Question
Question: An oxide ($Fe_2O_n$) of iron contains 70% iron by mass. Calculate value of *n*....
An oxide (Fe2On) of iron contains 70% iron by mass. Calculate value of n.
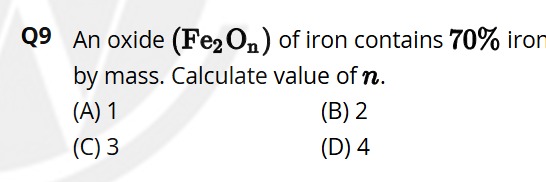
1
2
3
4
The value of n is 3.
Solution
The given oxide of iron has the formula Fe2On. It contains 70% iron by mass. We need to find the value of n.
The molar mass of Fe is approximately 56 g/mol, and the molar mass of O is approximately 16 g/mol.
The mass percentage of iron in the compound Fe2On is given by: Mass % of Fe = Molar mass of Fe2OnMass of 2 Fe atoms×100% Mass % of Fe = 2×MFe+n×MO2×MFe×100%
Given that the mass percentage of Fe is 70%, we have: 70=2×56+n×162×56×100 70=112+16n112×100
Divide both sides by 100: 0.70=112+16n112
Rearrange the equation to solve for n: 0.70×(112+16n)=112 0.70×112+0.70×16n=112 78.4+11.2n=112
Subtract 78.4 from both sides: 11.2n=112−78.4 11.2n=33.6
Divide by 11.2: n=11.233.6 n=112336 n=3
Thus, the value of n is 3. The oxide is Fe2O3.
The mass percentage of iron in Fe2On is given by (2×MFe)/(2×MFe+n×MO). Setting this equal to 70% and using atomic masses MFe=56 and MO=16, we get the equation 0.70=112+16n112. Solving this equation for n yields n=3.
