Question
Question: A solution was prepared by dissolving 2g of a non-volatile non electrolyte solid **B** in 100g of so...
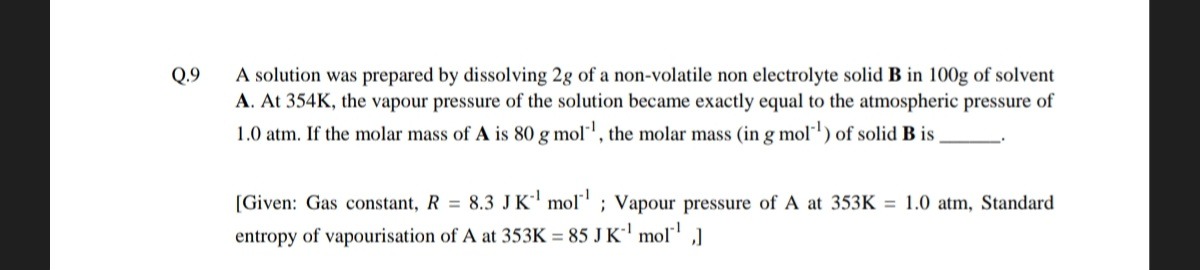
A solution was prepared by dissolving 2g of a non-volatile non electrolyte solid B in 100g of solvent A. At 354K, the vapour pressure of the solution became exactly equal to the atmospheric pressure of 1.0 atm. If the molar mass of A is 80 gmol−1, the molar mass (in gmol−1) of solid B is ____.
[Given: Gas constant, R=8.3J K−1mol−1; Vapour pressure of A at 353K = 1.0 atm, Standard entropy of vapourisation of A at 353K = 85 J K−1mol−1,]
55.1
Solution
Here's how to solve this problem using the concept of boiling point elevation:
-
Determine the boiling point elevation (ΔTb) from the boiling points of the pure solvent and the solution: ΔTb=Tsolution−Tsolvent=354 K−353 K=1 K
-
Calculate the molar enthalpy of vaporization (ΔHvap) of the solvent using the given standard entropy of vaporization (ΔSvap) and the boiling point of the solvent (Tb): ΔHvap=Tb⋅ΔSvap=353 K⋅85 J K−1mol−1=30005 J mol−1
-
Calculate the molal elevation constant (Kb) using the formula: Kb=1000⋅ΔHvapR⋅Tb2⋅MA Where:
- R=8.3 J K−1mol−1
- Tb=353 K
- MA=80 g mol−1
- ΔHvap=30005 J mol−1
Kb=1000⋅300058.3⋅(353)2⋅80≈2.7545 K kg mol−1
-
Use the boiling point elevation equation to find the molality (m) of the solution: ΔTb=Kb⋅m 1 K=2.7545 K kg mol−1⋅m m≈0.3630 mol kg−1
-
Use the definition of molality to calculate the molar mass of the solute (MB): m=kg of solventmoles of solute=wAwB/MB Where:
- wB=2 g
- wA=100 g=0.1 kg
0.3630=0.12/MB MB=0.3630⋅0.12≈55.09 g mol−1
Therefore, the molar mass of solid B is approximately 55.1 gmol−1.
