Question
Question: The reaction most likely occurs by which of the following mechanism?...
The reaction most likely occurs by which of the following mechanism?
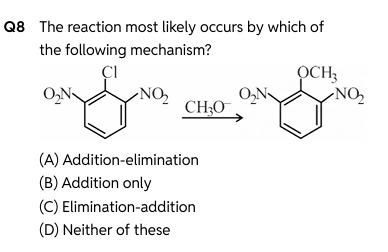
Addition-elimination
Addition only
Elimination-addition
Neither of these
Addition-elimination
Solution
The given reaction is a nucleophilic aromatic substitution. The substrate is 1-chloro-2,4-dinitrobenzene, which has two strong electron-withdrawing nitro (-NO₂) groups at the ortho and para positions relative to the chlorine atom. The reagent is methoxide ion (CH₃O⁻), a strong nucleophile.
Aryl halides generally do not undergo nucleophilic substitution easily. However, the presence of strong electron-withdrawing groups at ortho or para positions activates the benzene ring towards nucleophilic attack. This reaction proceeds via the addition-elimination mechanism, also known as the SNAr (nucleophilic aromatic substitution) mechanism.
The mechanism involves two main steps:
-
Addition: The nucleophile (CH₃O⁻) attacks the carbon atom bearing the leaving group (Cl). This attack is facilitated by the electron-withdrawing nitro groups, which delocalize and stabilize the negative charge that develops on the ring in the intermediate. This forms a resonance-stabilized intermediate called a Meisenheimer complex (or sigma complex). In the Meisenheimer complex, the carbon atom that was attacked becomes sp³ hybridized, temporarily breaking the aromaticity. The negative charge is delocalized onto the ortho and para carbons, and effectively onto the oxygen atoms of the nitro groups via resonance.
-
Elimination: The leaving group (Cl⁻) departs from the Meisenheimer complex, which restores aromaticity and forms the substituted product.
The elimination-addition mechanism (benzyne mechanism) typically occurs with aryl halides that lack strong electron-withdrawing groups or under very harsh conditions (e.g., NaNH₂ in liquid NH₃), and often leads to isomeric products. Given the presence of strong activating groups, the addition-elimination mechanism is overwhelmingly favored here.
