Question
Question: A certain radioactive nuclide of mass number $m_x$ disintegrates, with the emission of an electron a...
A certain radioactive nuclide of mass number mx disintegrates, with the emission of an electron and γ radiation only, to give second nuclied of mass number my. Which one of the following equation correctly relates mx and my?
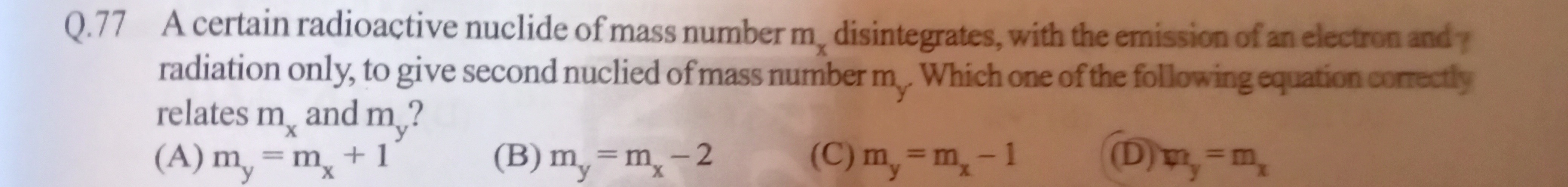
m_y = m_x + 1
m_y = m_x - 2
m_y = m_x - 1
m_y = m_x
m_y = m_x
Solution
The radioactive decay described involves the emission of an electron, which is characteristic of beta-minus (β−) decay. In β− decay, a neutron within the nucleus transforms into a proton, an electron (emitted as a beta particle), and an antineutrino. Gamma (γ) radiation is electromagnetic radiation and does not affect the mass number or atomic number of the nucleus.
The transformation of a neutron into a proton can be represented as: n→p+e−+νˉe
Let the initial nuclide be denoted by X with mass number mx and atomic number Zx. Let the resulting nuclide be Y with mass number my and atomic number Zy. The decay process can be written as: ZxmxX→ZymyY+e−+γ
In this process, the number of nucleons (protons + neutrons) remains constant. A neutron is replaced by a proton, but the total count of these particles stays the same. Therefore, the mass number (m) of the nuclide does not change.
Thus, the mass number of the initial nuclide (mx) is equal to the mass number of the final nuclide (my). my=mx
