Question
Question: Select the correct sequence of increasing radius (left to right)...
Select the correct sequence of increasing radius (left to right)
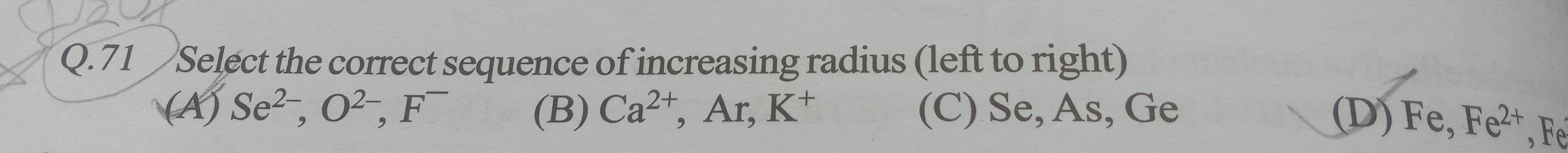
A
Se2−, O2−, F−
B
Ca2+, Ar, K+
C
Se, As, Ge
D
Fe, Fe2+, Fe
Answer
Se, As, Ge
Explanation
Solution
- (A) Se2−, O2−, F−: O2− and F− are isoelectronic species. O2− (Z=8) has a lower nuclear charge than F− (Z=9), so O2− is larger than F−. Se2− is in Period 4, while O2− and F− are in Period 2. Radii increase down a group, so Se2− is larger than O2− and F−. The correct order is F− < O2− < Se2−. Option (A) is incorrect.
- (B) Ca2+, Ar, K+: These are isoelectronic species with 18 electrons. Ca2+ (Z=20), K+ (Z=19), Ar (Z=18). Radius decreases with increasing nuclear charge. The correct order of increasing radius is Ca2+ < K+ < Ar. Option (B) is incorrect.
- (C) Se, As, Ge: These elements are in Period 4. Ge (Group 14), As (Group 15), Se (Group 16). Atomic radius generally decreases across a period from left to right. Thus, Radius(Ge) > Radius(As) > Radius(Se). The correct sequence of increasing radius is Se, As, Ge. Option (C) is correct.
- (D) Fe, Fe2+, Fe: A cation is smaller than its parent atom. Fe2+ is smaller than Fe. The sequence Fe, Fe2+, Fe is incorrect because Radius(Fe) > Radius(Fe2+).
