Question
Question: A perfect gas (0.1 mol) having $\bar{C}_v$ = 1.50R (independent of temperature) undergoes the above ...
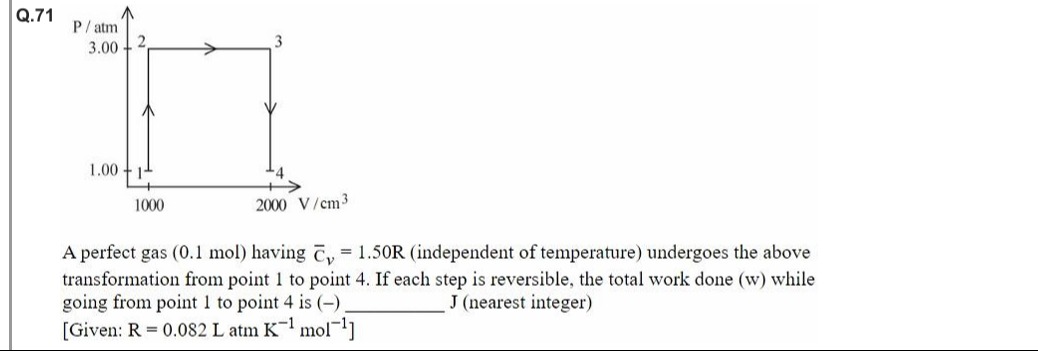
A perfect gas (0.1 mol) having Cˉv = 1.50R (independent of temperature) undergoes the above transformation from point 1 to point 4. If each step is reversible, the total work done (w) while going from point 1 to point 4 is (-) _______ J (nearest integer)
[Given: R = 0.082 L atm K−1 mol−1]
Answer
-304 J
Explanation
Solution
-
Identify the processes:
- 1 → 2: Isochoric (constant V); no work done.
- 2 → 3: Isobaric (constant P); work done w=PΔV.
- 3 → 4: Isochoric; no work done.
-
Calculate work for process 2 → 3:
- At state 2: V=1000cm3=1L
- At state 3: V=2000cm3=2L
- Pressure P=3.00atm
- Work (in L·atm): w2→3=3.00atm×(2L−1L)=3.00L\cdotpatm
-
Convert work into Joules:
- Use conversion: 1L\cdotpatm=101.325J w2→3=3.00×101.325J≈303.975J
- The gas expands in process 2 → 3, so by convention the work done by the system is negative: w=−304J(nearest integer)
Explanation (Minimal Core):
Only process 2 → 3 does work: w=PΔV=3atm×1L=3L\cdotpatm=303.975J, and with expansion, w is negative, so w≈−304J.
