Question
Question: Vapour pressure of pure A = 100 torr, moles = 2; vapour pressure of pure B = 80 torr, moles = 3. Tot...
Vapour pressure of pure A = 100 torr, moles = 2; vapour pressure of pure B = 80 torr, moles = 3. Total vapour pressure of the mixture is
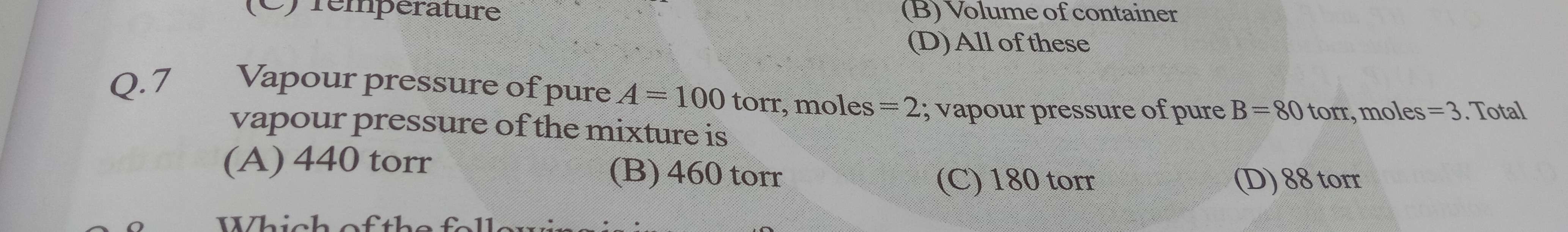
440 torr
460 torr
180 torr
88 torr
88 torr
Solution
Here's how to calculate the total vapor pressure of the mixture:
-
Calculate Mole Fractions in Liquid Phase:
Total moles of the mixture (ntotal) = Moles of A (nA) + Moles of B (nB)
ntotal=2 moles+3 moles=5 moles
Mole fraction of A (XA) = ntotalnA=52=0.4
Mole fraction of B (XB) = ntotalnB=53=0.6
-
Calculate Partial Pressures in Vapor Phase (Raoult's Law):
According to Raoult's Law, the partial pressure of a component in an ideal solution is given by Pi=XiPi0, where Pi0 is the vapor pressure of the pure component.
Partial pressure of A (PA) = XA⋅PA0=0.4×100 torr=40 torr
Partial pressure of B (PB) = XB⋅PB0=0.6×80 torr=48 torr
-
Calculate Total Vapor Pressure (Dalton's Law of Partial Pressures):
The total vapor pressure of the mixture (Ptotal) is the sum of the partial pressures of its components.
Ptotal=PA+PB=40 torr+48 torr=88 torr
