Question
Question: The alkene formed as a major product in the below elimination reaction is ...
The alkene formed as a major product in the below elimination reaction is
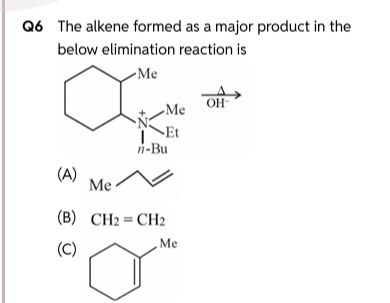
Me
CH2=CH2
CH₂=CH₂
Solution
The given reaction is a Hofmann elimination, which is a type of elimination reaction undergone by quaternary ammonium salts upon heating with a strong base (like OH⁻).
Hofmann Elimination Rule:
In Hofmann elimination, the major alkene product is the one that is least substituted (i.e., has the fewest alkyl substituents on the double bond carbons). This typically corresponds to the removal of a β-hydrogen from the β-carbon that has the most hydrogen atoms. This is contrary to Zaitsev's rule, which favors the more substituted alkene.
Step-by-step analysis of the reactant and possible β-hydrogens:
The reactant is a quaternary ammonium salt, specifically a derivative of 2-methylcyclohexylamine. The nitrogen atom is positively charged and is bonded to four carbon groups:
- A methyl group (Me)
- An ethyl group (Et, -CH₂CH₃)
- An n-butyl group (n-Bu, -CH₂CH₂CH₂CH₃)
- A 2-methylcyclohexyl group (the ring system)
We need to identify all possible β-carbons (carbons adjacent to the α-carbon, which is the carbon directly attached to the positively charged nitrogen) and the number of β-hydrogens on each.
-
From the Methyl (Me) group attached to Nitrogen:
The carbon is directly attached to N. There are no β-carbons or β-hydrogens on this group that can undergo elimination.
-
From the Ethyl (Et) group (-CH₂CH₃):
The α-carbon (attached to N) is -CH₂-. The β-carbon is the terminal -CH₃.
Number of β-hydrogens = 3 (from -CH₃).
Potential alkene product: CH₂=CH₂ (Ethene)
-
From the n-Butyl (n-Bu) group (-CH₂CH₂CH₂CH₃):
The α-carbon (attached to N) is the first -CH₂-. The β-carbon is the second -CH₂-.
Number of β-hydrogens = 2 (from the second -CH₂-).
Potential alkene product: CH₂=CH-CH₂CH₃ (1-Butene)
-
From the 2-methylcyclohexyl group:
Let's consider the carbon atom of the ring directly attached to the nitrogen as C1 (α-carbon).
-
β-carbon at C2: This carbon has a methyl group and one hydrogen atom.
Number of β-hydrogens = 1.
Potential alkene product: 1-methylcyclohexene (double bond between C1 and C2).
-
β-carbon at C6: This carbon has two hydrogen atoms.
Number of β-hydrogens = 2.
Potential alkene product: Cyclohexene (double bond between C1 and C6).
-
Applying Hofmann's Rule to determine the major product:
According to Hofmann's rule, the major product is formed by the removal of a β-hydrogen from the β-carbon that has the most hydrogen atoms. Let's compare the number of β-hydrogens for each possible elimination:
- Ethyl group: 3 β-hydrogens (leading to Ethene)
- n-Butyl group: 2 β-hydrogens (leading to 1-Butene)
- Cyclohexyl ring (C6): 2 β-hydrogens (leading to Cyclohexene)
- Cyclohexyl ring (C2): 1 β-hydrogen (leading to 1-methylcyclohexene)
The β-carbon with the highest number of hydrogens is the terminal methyl group of the ethyl substituent (3 β-hydrogens). Therefore, the elimination of these hydrogens will lead to the major product.
The major product formed is ethene (CH₂=CH₂).
