Question
Question: Which of the following is called negative overlap?...
Which of the following is called negative overlap?
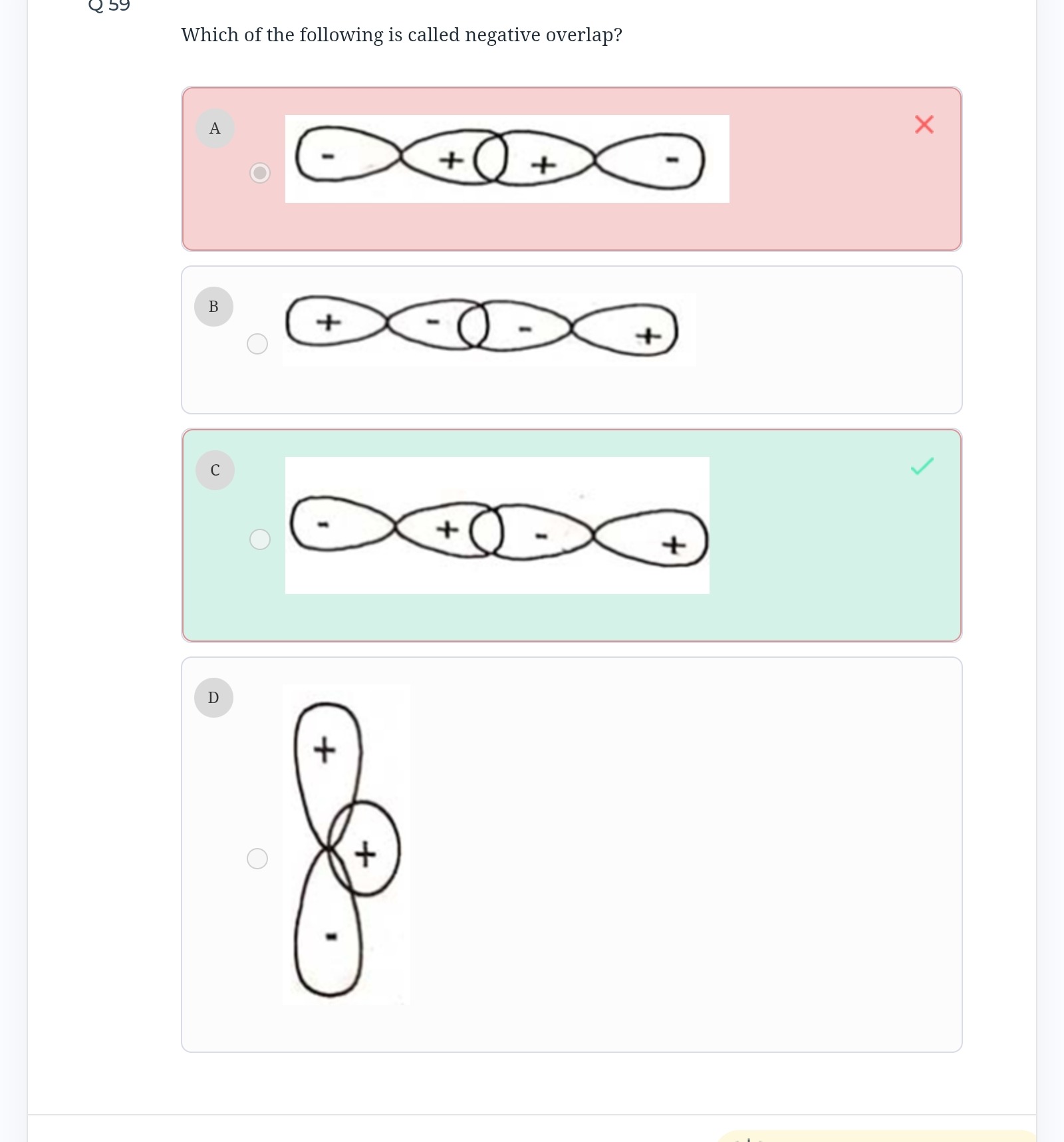
A
X
B
C
D
Answer
C
Explanation
Solution
In the context of atomic orbital overlap, the signs (+ and -) on the lobes of the orbitals represent the phase of the wave function.
- Positive Overlap (Constructive Interference): Occurs when two atomic orbitals with the same phase overlap. This leads to an increase in electron density between the nuclei and forms a bonding molecular orbital. Examples include (+) overlapping with (+) or (-) overlapping with (-).
- Negative Overlap (Destructive Interference): Occurs when two atomic orbitals with opposite phases overlap. This leads to a decrease in electron density between the nuclei, forming a nodal plane, and results in an antibonding molecular orbital. This is represented by (+) overlapping with (-).
- Zero Overlap: Occurs when there is no net overlap due to the orientation of orbitals, or when positive and negative overlaps cancel each other out due to symmetry. This results in a non-bonding interaction.
Let's analyze each option:
- A: Shows the overlap between a '+' lobe of one p-orbital and a '+' lobe of another p-orbital. This is a positive overlap.
- B: Shows the overlap between a '-' lobe of one p-orbital and a '-' lobe of another p-orbital. This is also a positive overlap.
- C: Shows the overlap between a '+' lobe of one p-orbital and a '-' lobe of another p-orbital. This represents the overlap of opposite phases, which is a negative overlap. This type of overlap leads to the formation of an antibonding molecular orbital.
- D: Shows an s-orbital overlapping side-on with a p-orbital. The s-orbital (represented by a '+' sign) overlaps with both the '+' and '-' lobes of the p-orbital. Due to the symmetry of this arrangement, the positive overlap between the s-orbital and the '+' lobe of the p-orbital is cancelled out by the negative overlap between the s-orbital and the '-' lobe of the p-orbital. Therefore, the net overlap is zero, leading to a non-bonding interaction. This is not typically referred to as "negative overlap" in the sense of forming an antibonding orbital through direct destructive interference between specific lobes.
Based on the definitions, Option C clearly depicts negative overlap.
