Question
Question: In the given reaction : ...
In the given reaction :
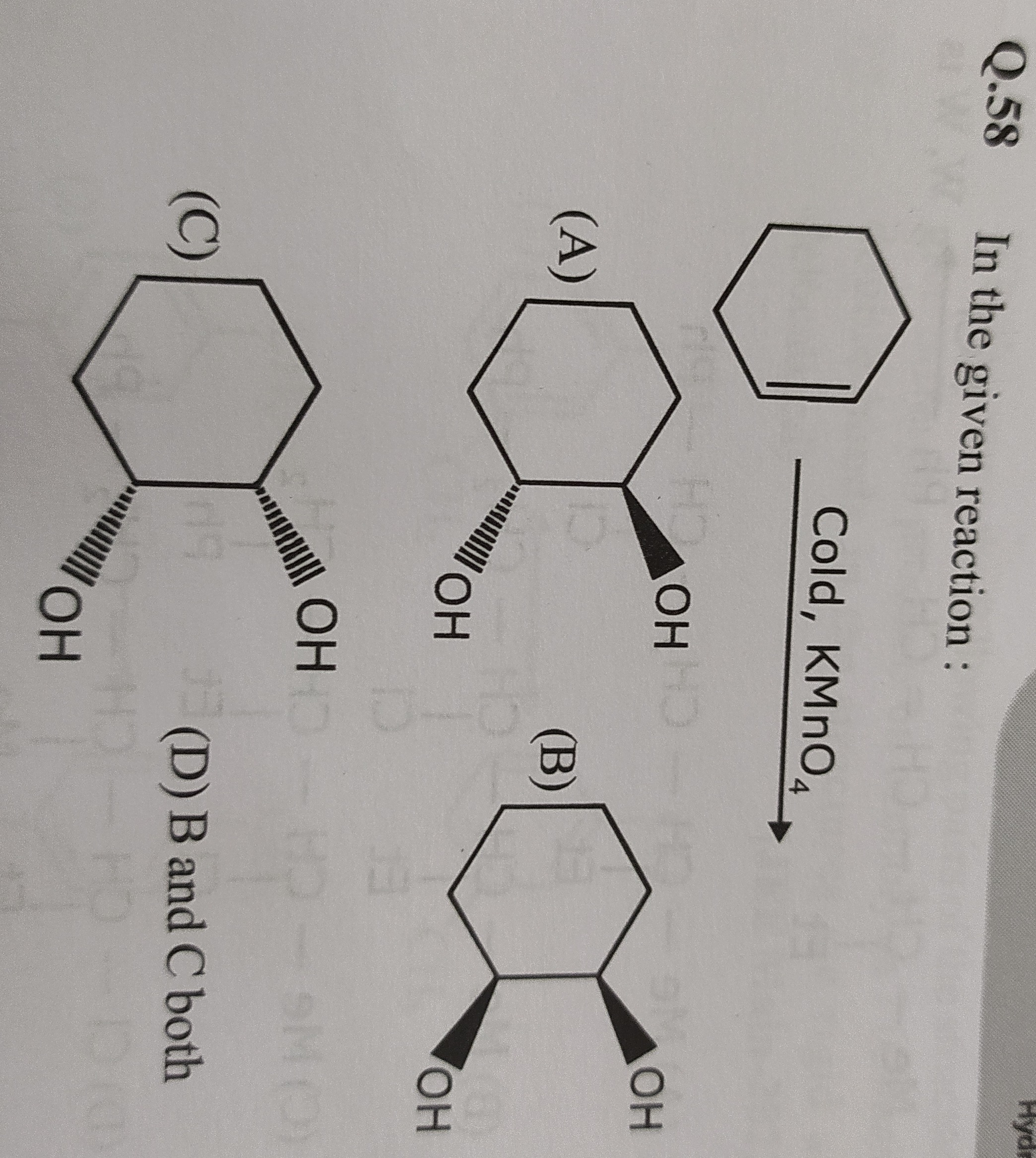
A
(A)
B
(B)
C
(C)
D
(D) B and C both
Answer
D
Explanation
Solution
The reaction given is the treatment of cyclohexene with cold, dilute potassium permanganate (KMnO4), also known as Baeyer's reagent.
- Nature of Reagent: Cold, dilute, alkaline KMnO4 is a mild oxidizing agent used for the hydroxylation of alkenes. It is specifically known to cause syn-dihydroxylation.
- Syn-dihydroxylation: This means that two hydroxyl (-OH) groups are added to the carbons of the double bond from the same face (or side) of the alkene.
- Product of Cyclohexene: When cyclohexene undergoes syn-dihydroxylation, the double bond breaks, and two -OH groups are added to the adjacent carbon atoms. Since the addition is syn, both -OH groups will be on the same side of the cyclohexane ring. This product is cis-cyclohexane-1,2-diol.
Since the starting material (cyclohexene) is achiral and the reagent (cold KMnO4) is also achiral, the reaction will yield a racemic mixture of two enantiomers in equal amounts. Therefore, both B and C are formed as products.
- Option A shows a trans-1,2-diol (one -OH is wedge, one is dash), which would result from anti-dihydroxylation, not syn-dihydroxylation by Baeyer's reagent.
- Option B shows one enantiomer of the cis-1,2-diol.
- Option C shows the other enantiomer of the cis-1,2-diol.
- Option D correctly states that both B and C are formed.
The final answer is D
