Question
Question: When undergoes intramolecular aldol condensation, the major product formed is :...
When undergoes intramolecular aldol condensation, the major product formed is :
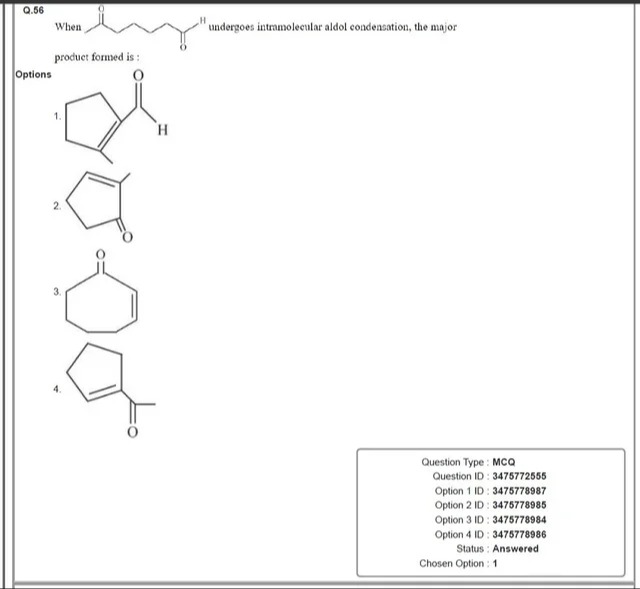
A five-membered ring with a double bond between carbon 3 and carbon 4. A carbonyl group is attached to carbon 1, forming an aldehyde.
A five-membered ring with a double bond between carbon 3 and carbon 4. A carbonyl group is attached to carbon 2, forming a ketone.
A six-membered ring with a double bond between carbon 4 and carbon 5. A carbonyl group is attached to carbon 1, forming a ketone.
A five-membered ring with a double bond between carbon 3 and carbon 4. An acetyl group (COCH3) is attached to carbon 2, forming a ketone.
A six-membered ring with a double bond between carbon 4 and carbon 5. A carbonyl group is attached to carbon 1, forming a ketone.
Solution
For a 1,7-diketone (O=C–(CH₂)₅–C=O), the most acidic α‐hydrogens are at C2 (and symmetrically C6). When an enolate ion forms at C2, it attacks the distal C7 carbonyl intramolecularly. This cyclization produces a six‐membered ring. After subsequent dehydration, a cyclohexenone is formed with the carbonyl group retained on the ring. This is the stable product of the intramolecular aldol condensation.
