Question
Question: Consider the following compound (X) $\mathrm{H}-\stackrel{\mathrm{I}}{\mathrm{C}}=\stackrel{\mathrm...
Consider the following compound (X)
H−CI=CII−CH2III−CHCH−CH3IV
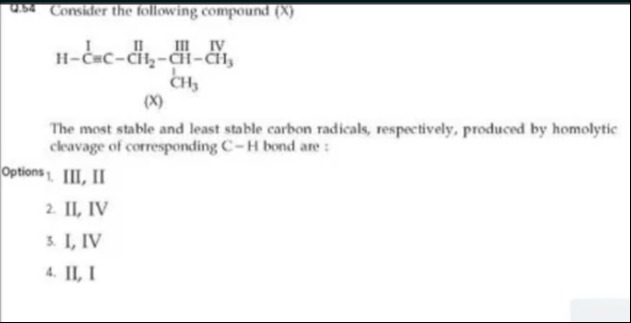
Answer
There are two stereoisomers of (X): the (E)- and (Z)-isomers of 1,4‑pentadiene.
Explanation
Solution
Solution:
We analyze the structure given by
H−CI=CII−CH2III−CH=CH3IVRewriting this in a more conventional form, we have
CH2=CH−CH2−CH=CHCH3.Step 1. Identify the double bonds:
- The first double bond is at CH2=CH−. Here the left carbon is CH2 (two hydrogens) so it cannot exhibit cis–trans isomerism.
- The second double bond is at −CH=CHCH3. On the left carbon of this double bond, one group is the CH2 (from the adjoining −CH2−) and the other is a hydrogen. On the right carbon, one substituent is a methyl group (CH3) and the other is a hydrogen. Since both carbons have two different groups, this double bond can exist in two geometric forms (E and Z).
Step 2. Conclusion:
Thus, the molecule is 1,4‑pentadiene. Only the second double bond is stereogenic, giving rise to two geometric isomers: the (E)- and (Z)-1,4-pentadiene.
For a visual representation in mermaid:
Minimal Explanation of the Core Steps:
- Rewrite the structure as CH2=CH−CH2−CH=CHCH3.
- Note that the terminal double bond (C₁=C₂) has a CH₂ group (no isomerism).
- Recognize that the internal double bond (C₄=C₅) has two different substituents on each sp² carbon.
- Conclude that two stereoisomers ((E) and (Z)) are possible.
