Question
Question: Electronic configuration of four elements A, B, C and D are given below: (A) 1s$^2$2s$^2$2p$^3$ (B) ...
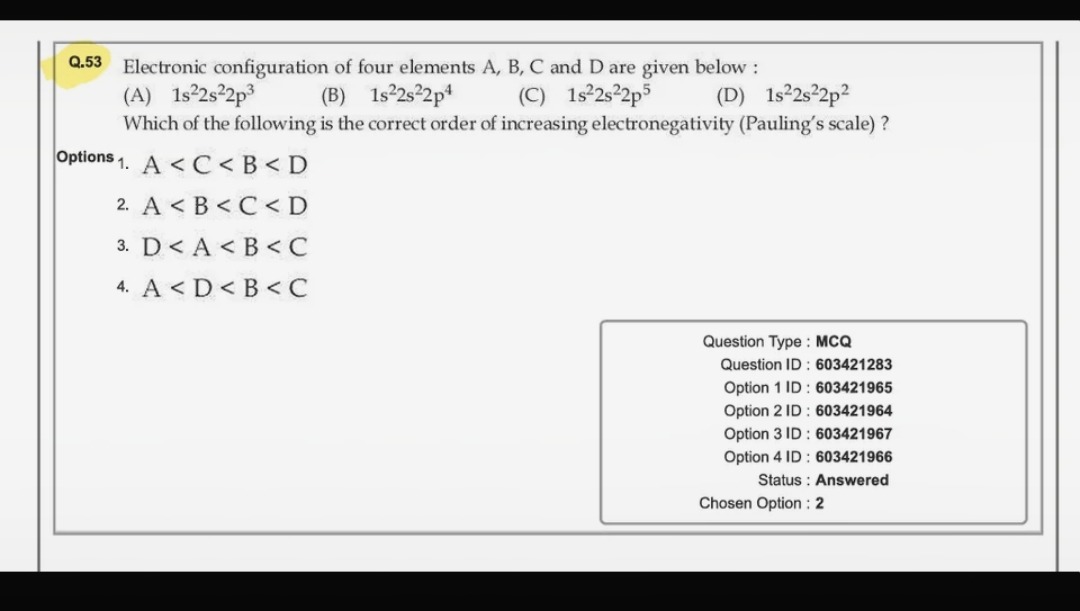
Electronic configuration of four elements A, B, C and D are given below: (A) 1s22s22p3 (B) 1s22s22p4 (C) 1s22s22p5 (D) 1s22s22p2 Which of the following is the correct order of increasing electronegativity (Pauling's scale)?
A
A < C < B < D
B
A < B < C < D
C
D < A < B < C
D
A < D < B < C
Answer
D < A < B < C
Explanation
Solution
-
Identify the elements from their electronic configurations:
- A: 1s2 2s2 2p3 → Nitrogen (N)
- B: 1s2 2s2 2p4 → Oxygen (O)
- C: 1s2 2s2 2p5 → Fluorine (F)
- D: 1s2 2s2 2p2 → Carbon (C)
-
Using electronegativity values (Pauling scale):
- C (Carbon): ~2.55
- N (Nitrogen): ~3.04
- O (Oxygen): ~3.44
- F (Fluorine): ~3.98
-
Increasing order (from lowest to highest): Carbon < Nitrogen < Oxygen < Fluorine → D < A < B < C.
