Question
Question: For the reaction A + B → C; starting with different initial concentration of A and B, initial rate o...
For the reaction A + B → C; starting with different initial concentration of A and B, initial rate of reaction were determined graphically in four experiments.
Rate law for reaction from above data is
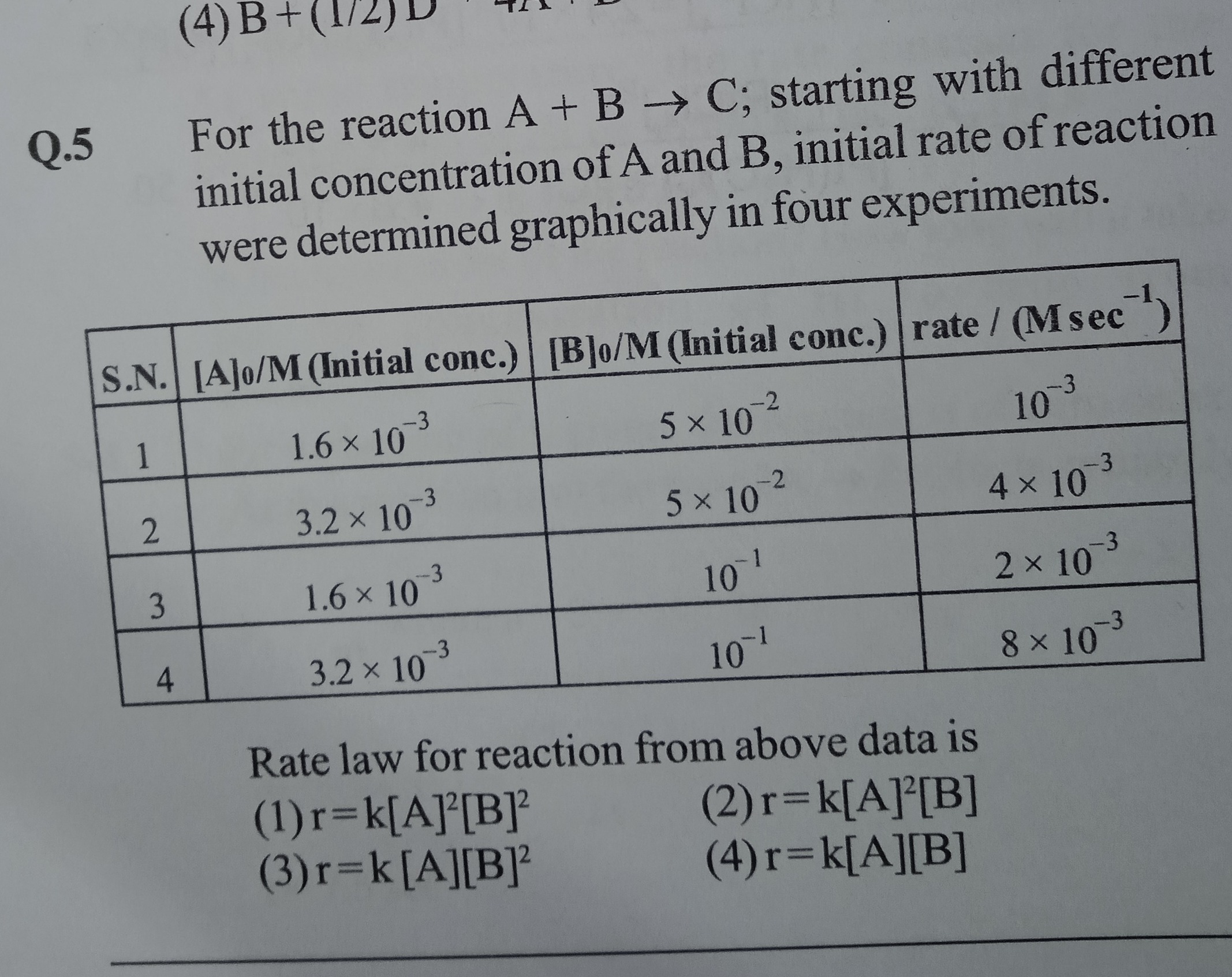
r=k[A]²[B]²
r=k[A][B]²
r=k[A]²[B]
r=k[A][B]
r=k[A]²[B]
Solution
To determine the rate law, we analyze how the initial concentrations of A and B affect the initial reaction rate.
-
Determining the order with respect to A:
Compare Experiment 1 and Experiment 2 (only [A] changes):\frac{[A]_2}{[A]_1} = \frac{3.2\times10^{-3}}{1.6\times10^{-3}} = 2 \quad \text{and} \quad \frac{\text{Rate}_2}{\text{Rate}_1} = \frac{4\times10^{-3}}{1\times10^{-3}} = 4.Since 2x=4, x=2. Hence, the reaction is second order in A.
-
Determining the order with respect to B:
Compare Experiment 1 and Experiment 3 (only [B] changes):\frac{[B]_3}{[B]_1} = \frac{1.0\times10^{-1}}{5\times10^{-2}} = 2 \quad \text{and} \quad \frac{\text{Rate}_3}{\text{Rate}_1} = \frac{2\times10^{-3}}{1\times10^{-3}} = 2.Since 2y=2, y=1. Hence, the reaction is first order in B.
-
Rate Law:
Combining the orders, the rate law is:\text{rate} = k[A]^2[B].Therefore, the rate law is r=k[A]2[B].
