Question
Question: An electron in the hydrogen atom initially in the fourth excited state makes a transition to n$^{th}...
An electron in the hydrogen atom initially in the fourth excited state makes a transition to nth energy state by emitting a photon of energy 2.86 eV. The integer value of n will be _________.
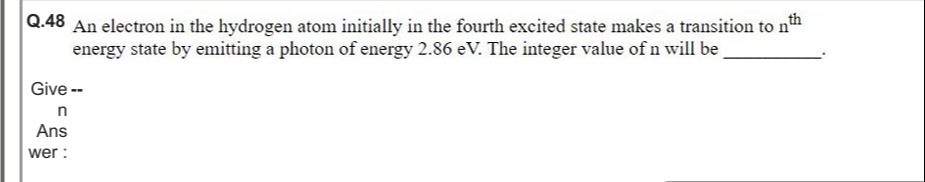
Answer
2
Explanation
Solution
-
For a hydrogen atom, the energy is given by
En=−n213.6 eV.
-
The fourth excited state corresponds to ni=5 (since ground state n=1).
-
Energy of electron in initial state:
E5=−2513.6≈−0.544 eV.
-
Let the final state be nf=n. The energy emitted during the transition is:
Eγ=E5−En=2.86 eV.
-
So,
En=E5−2.86≈−0.544−2.86=−3.404 eV.
-
Equate with the energy level expression:
−n213.6=−3.404
n213.6=3.404
n2=3.40413.6=4
n=2.
Use En=−n213.6 and compute the energy difference E5−En=2.86 eV. Solve for n.
