Question
Question: In the reaction with HCl, an alkene reacts in accordance with the Markovnikov's rule, to give a prod...
In the reaction with HCl, an alkene reacts in accordance with the Markovnikov's rule, to give a product 1-chloro-1-methylcyclohexane. The possible alkene is ____________________________.
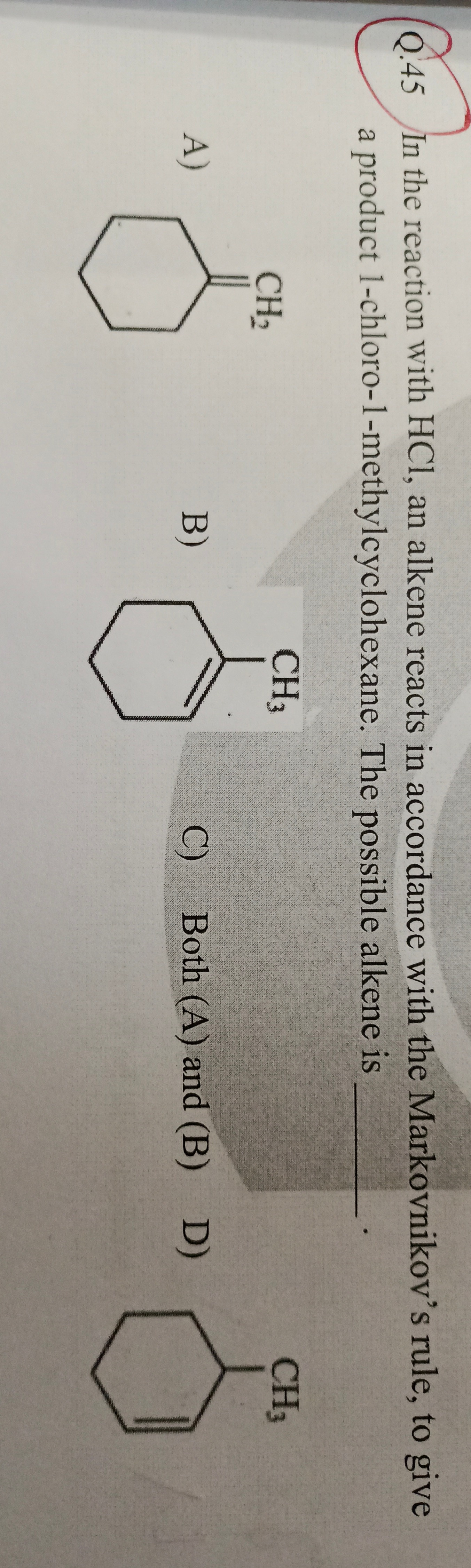
A
A)
B
B)
C
Both (A) and (B)
D
D)
Answer
Both (A) and (B)
Explanation
Solution
The product 1-chloro-1-methylcyclohexane has a chlorine and a methyl group on the same carbon, which is a tertiary carbon. According to Markovnikov's rule, the halogen (Cl) adds to the more substituted carbon of the double bond, leading to the formation of the most stable carbocation (tertiary in this case).
- Methylenecyclohexane (Option A): The double bond is between the ring carbon and the exocyclic methylene group. The ring carbon is more substituted (has 0 hydrogens) than the exocyclic carbon (which has 2 hydrogens). Upon addition of H+, H adds to the CH2, forming a CH3 group, and a tertiary carbocation forms on the ring carbon. Subsequent addition of Cl- at this tertiary carbon yields 1-chloro-1-methylcyclohexane.
- 1-methylcyclohexene (Option B): The double bond is between the carbon bearing the methyl group (which has 0 hydrogens) and an adjacent carbon (which has 1 hydrogen). Upon addition of H+, H adds to the carbon with 1 hydrogen, and a tertiary carbocation forms on the carbon bearing the methyl group. Subsequent addition of Cl- at this tertiary carbon yields 1-chloro-1-methylcyclohexane.
- 3-methylcyclohexene (Option D): The double bond is not directly adjacent to the methyl group. Addition of H+ would lead to secondary carbocations, which upon rearrangement would lead to products other than 1-chloro-1-methylcyclohexane (e.g., 3-chloro-3-methylcyclohexane).
Thus, both methylenecyclohexane and 1-methylcyclohexene can yield the desired product.
