Question
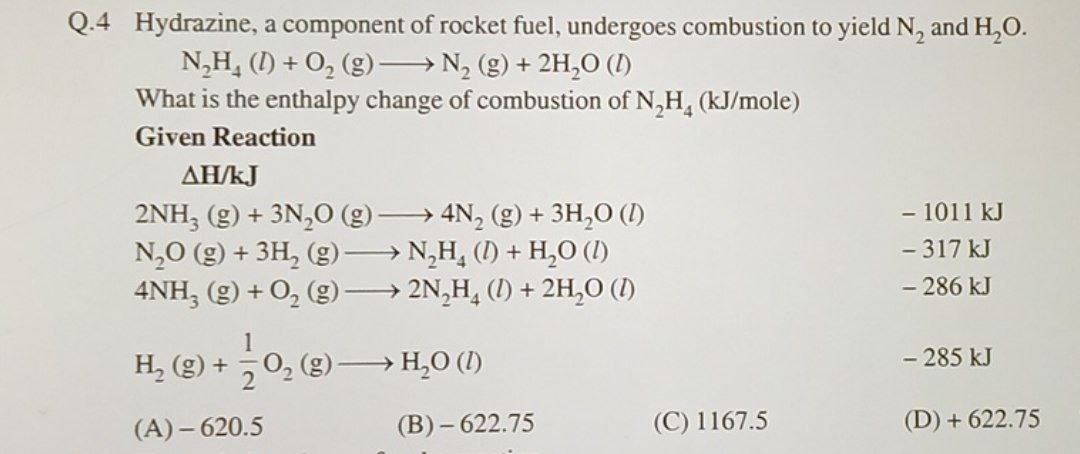
Question: Hydrazine, a component of rocket fuel, undergoes combustion to yield N₂ and H₂O. N₂H₄ (l) + O₂ (g) ...
Hydrazine, a component of rocket fuel, undergoes combustion to yield N₂ and H₂O.
N₂H₄ (l) + O₂ (g) → N₂ (g) + 2H₂O (l)
What is the enthalpy change of combustion of N₂H₄ (kJ/mole) Given Reaction ΔH/kJ 2NH₃ (g) + 3N₂O (g) → 4N₂ (g) + 3H₂O (l) - 1011 kJ N₂O (g) + 3H₂ (g) → N₂H₄ (l) + H₂O (l) - 317 kJ 4NH₃ (g) + O₂ (g) → 2N₂H₄ (l) + 2H₂O (l) - 286 kJ H₂(g) + 21O₂ (g) → H₂O (l) - 285 kJ
A
-620.5
B
-622.75
C
1167.5
D
- 622.75
Answer
-620.5 kJ/mole
Explanation
Solution
The enthalpy change of combustion of N₂H₄ can be calculated using Hess's Law and the given reactions.
-
From Reaction 1: 2x + 3y = 156
-
From Reaction 3: z = 2x + 142
-
From Reaction 2: z = y - 32
Solving these equations, we find:
- x = -45.75
- y = 82.5
- z = 50.5 kJ
Then, the combustion reaction ΔH is calculated as:
ΔH = [2(–285)] – (50.5) = –620.5 kJ.
