Question
Question: Two different masses m and 3m of an ideal gas are heated separately in a vessel of constant volume, ...
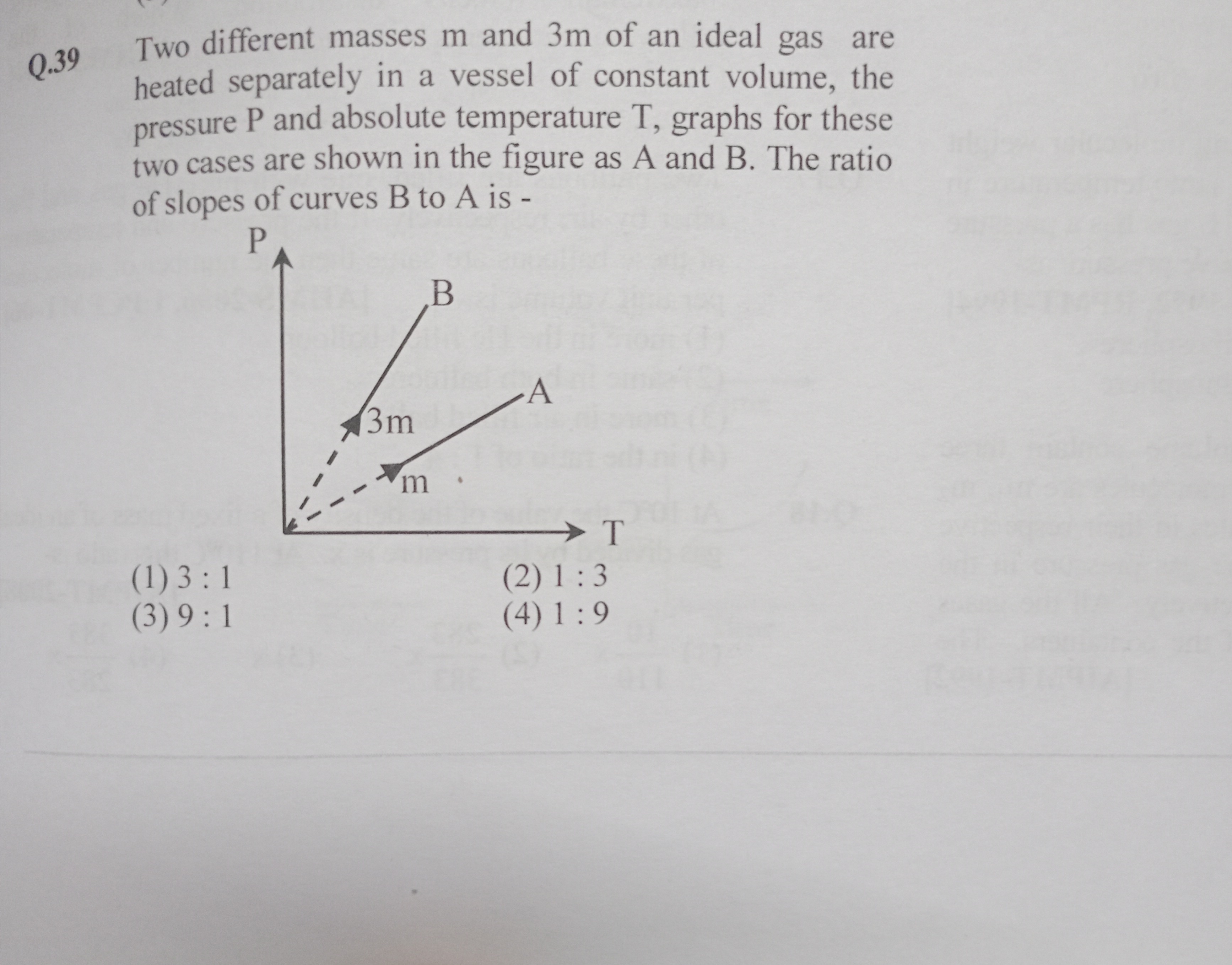
Two different masses m and 3m of an ideal gas are heated separately in a vessel of constant volume, the pressure P and absolute temperature T, graphs for these two cases are shown in the figure as A and B. The ratio of slopes of curves B to A is -
A
3 : 1
B
1 : 3
C
9 : 1
D
1 : 9
Answer
3 : 1
Explanation
Solution
For an ideal gas at constant volume:
P=VnRT⇒dTdP=VnRFor the sample with mass m, let the number of moles be n. For the sample with mass 3m, the number of moles is 3n. Therefore, the slopes of the P vs. T curves are proportional to n (and 3n respectively).
Thus, the ratio of the slopes (curve B corresponding to mass 3m to curve A corresponding to mass m) is:
n3n=3:1Brief Explanation:
At constant volume, dTdP∝n. For masses m and 3m, the mole ratio is 1:3, hence the slope ratio is 3:1.
