Question
Question: The major product in the following reaction is : H₃C CH=CH2 H₂O Heat ...
The major product in the following reaction is :
H₃C CH=CH2 H₂O Heat
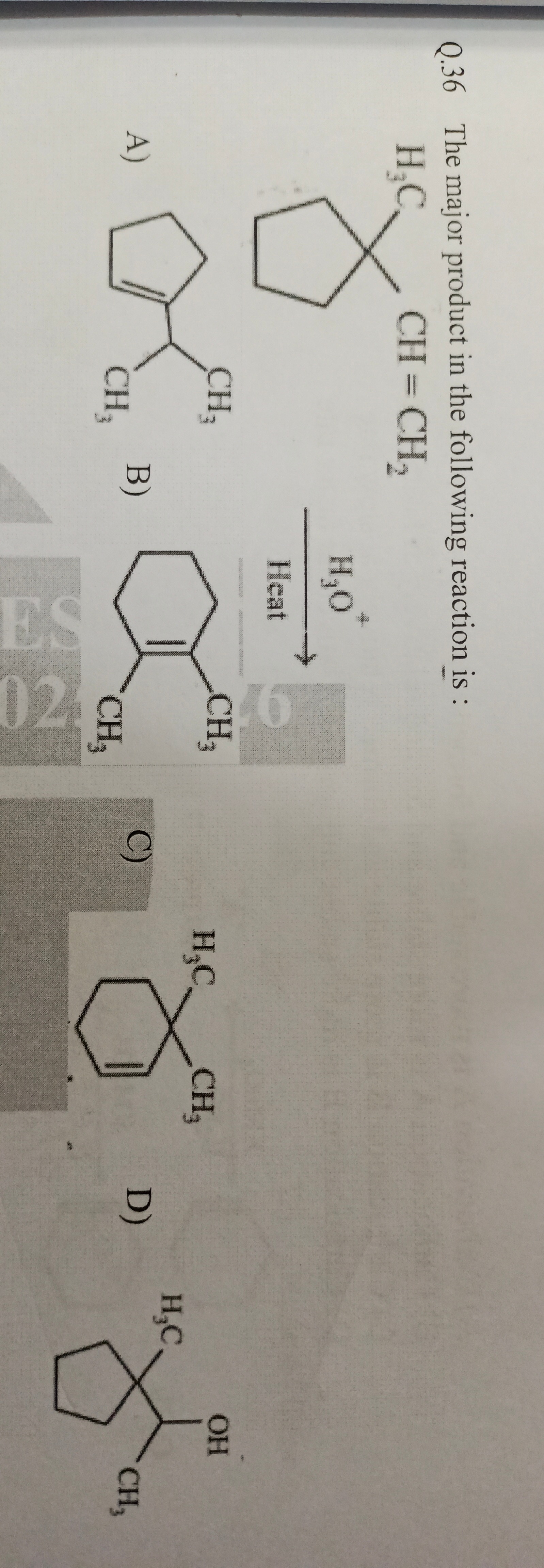
CH3
CH3 CH3
CH3 H₂C -CH₃ OH H3C
CH3
C
Solution
The reaction proceeds via acid-catalyzed hydration of the alkene to form a tertiary alcohol (2-cyclohexylpropan-2-ol) following Markovnikov's rule. This alcohol then undergoes dehydration under heating conditions (E1 elimination) to form the most stable alkene. The tertiary carbocation intermediate (2-cyclohexylpropan-2-yl carbocation) can eliminate a proton from either an adjacent methyl group or an adjacent carbon in the cyclohexyl ring. Elimination from the cyclohexyl ring forms 1-isopropylidenecyclohexane, which has a tetrasubstituted double bond, making it more stable (Zaitsev's rule) than the starting material (1-cyclohexyl-1-methylethene) which has a trisubstituted double bond. Thus, 1-isopropylidenecyclohexane is the major product.
