Question
Question: An optically active compound A undergoes first order conversion to another optically active compound...
An optically active compound A undergoes first order conversion to another optically active compounds B and C. From the following data of optical rotation, calculate the time from start at which the solution will become racemic mixture. A→B+C
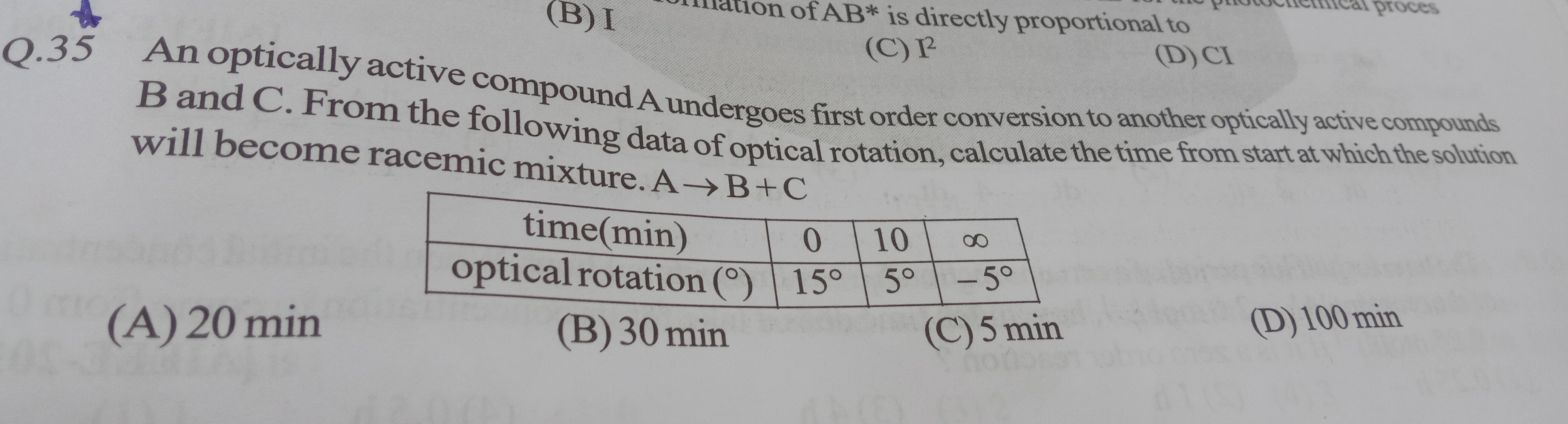
20 min
30 min
5 min
100 min
20 min
Solution
The reaction is a first-order conversion of an optically active compound A to other optically active compounds B and C: A → B + C.
The optical rotation at different times is given:
- Initial optical rotation at t = 0 min, r0=15∘
- Optical rotation at t = 10 min, rt=5∘
- Optical rotation at t = ∞ min (when the reaction is complete), r∞=−5∘
For a first-order reaction where the progress is monitored by optical rotation, the rate constant (k) is given by the formula:
k=t2.303log(rt−r∞r0−r∞)
First, let's calculate the rate constant (k) using the data at t = 10 min:
k=102.303log(5∘−(−5∘)15∘−(−5∘)) k=102.303log(5+515+5) k=102.303log(1020) k=102.303log(2)
Next, we need to find the time (t) at which the solution will become a racemic mixture. A racemic mixture has a net optical rotation of 0∘. So, we need to find t when rt=0∘.
Using the same first-order rate equation, rearrange it to solve for t:
t=k2.303log(rt−r∞r0−r∞)
Substitute the values: r0=15∘, r∞=−5∘, and rt=0∘. Also, substitute the expression for k we found:
t=(102.303log(2))2.303log(0∘−(−5∘)15∘−(−5∘)) t=log(2)10log(0+515+5) t=log(2)10log(520) t=log(2)10log(4)
We know that log(4)=log(22)=2log(2).
t=log(2)10(2log(2)) t=10×2 t=20 min
Thus, the solution will become a racemic mixture after 20 minutes from the start.
Explanation of the solution:
- Identify the reaction order and relevant formula: The problem states it's a first-order conversion, and data is given in terms of optical rotation. The rate constant (k) for a first-order reaction using optical rotation is k=t2.303log(rt−r∞r0−r∞).
- Calculate the rate constant (k): Use the given data (r0=15∘, r10=5∘, r∞=−5∘) to find k. k=102.303log(5−(−5)15−(−5))=102.303log(2).
- Determine the target optical rotation: A racemic mixture has an optical rotation of 0∘. So, we need to find t when rt=0∘.
- Calculate the time (t): Rearrange the first-order rate equation to solve for t, and substitute the known values including the calculated k. t=k2.303log(rt−r∞r0−r∞)=102.303log(2)2.303log(0−(−5)15−(−5))=log(2)10log(4). Since log(4)=2log(2), t=log(2)10(2log(2))=20 min.
