Question
Question: The results given in the below table were obtained during kinetic studies of the following reaction:...
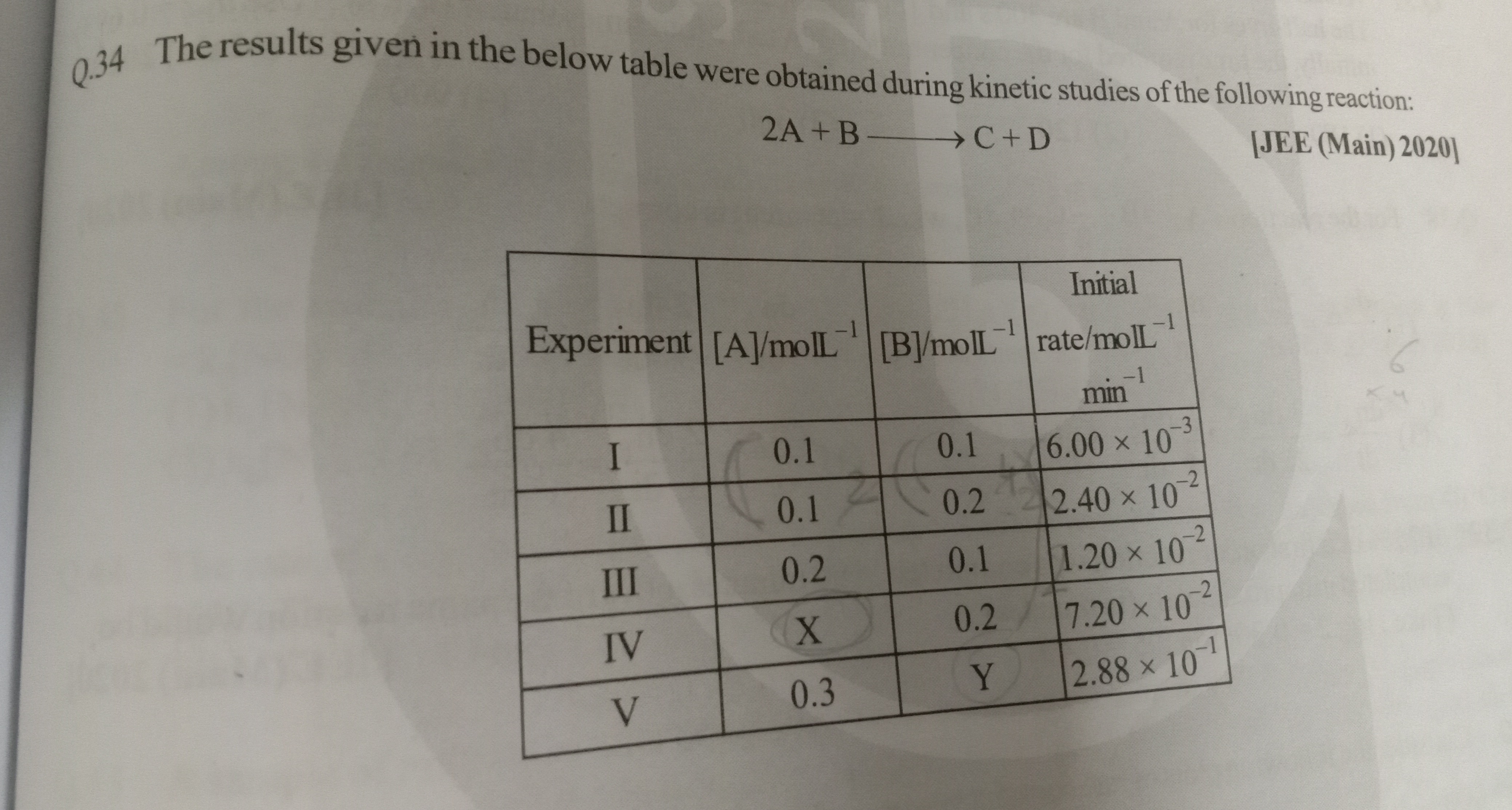
The results given in the below table were obtained during kinetic studies of the following reaction:
2A+B⟶C+D [JEE (Main) 2020]
| Experiment | [A]/molL−1 | [B]/molL−1 | Initial rate/molL−1 min−1 |
|---|---|---|---|
| I | 0.1 | 0.1 | 6.00×10−3 |
| II | 0.1 | 0.2 | 2.40×10−2 |
| III | 0.2 | 0.1 | 1.20×10−2 |
| IV | X | 0.2 | 7.20×10−2 |
| V | 0.3 | Y | 2.88×10−1 |
X=0.30, Y=0.40
Solution
The problem involves determining the rate law and then using it to find unknown concentrations.
-
Determine reaction orders: By comparing experiments where one reactant's concentration is kept constant while the other varies, the order of reaction with respect to each reactant is found.
- Comparing Exp I and II (constant [A]), doubling [B] quadruples the rate, indicating second order with respect to B (b=2).
- Comparing Exp I and III (constant [B]), doubling [A] doubles the rate, indicating first order with respect to A (a=1).
-
Write rate law: Combine the orders to get the overall rate law: Rate =k[A][B]2.
-
Calculate rate constant (k): Substitute data from any experiment (e.g., Exp I) into the rate law to find the value of k.
-
Calculate X and Y: Use the determined rate law and the calculated k value, along with the data from Experiments IV and V, to solve for the unknown concentrations X and Y.
