Question
Question: An ideal gas exists in a state with pressure $P_0$, volume $V_0$. It is isothermally expanded to 4 t...
An ideal gas exists in a state with pressure P0, volume V0. It is isothermally expanded to 4 times of its initial volume(V0), then isobarically compressed to its original volume. Finally the system is heated isochorically to bring it to its initial state. The amount of heat exchanged in this process is
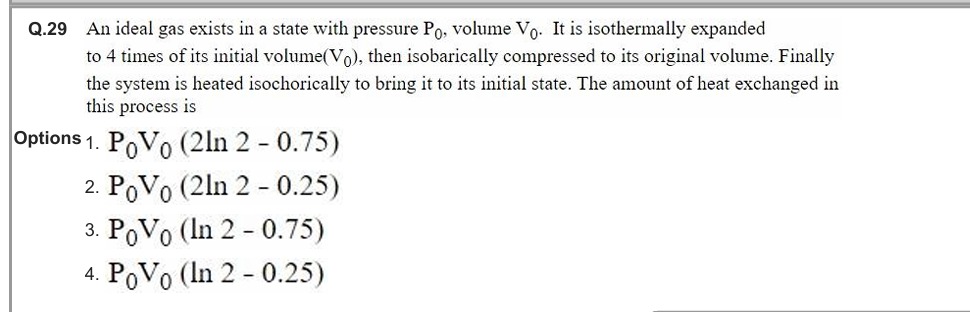
P0V0 (2ln 2 - 0.75)
P0V0 (2ln 2 - 0.25)
P0V0 (In 2 - 0.75)
P0V0 (In 2 - 0.25)
P0V0(2ln2−0.75)
Solution
We have a three‐step cyclic process for an ideal gas:
-
Isothermal Expansion from (P0,V0) to (P0/4,4V0):
For an isothermal process, ΔU=0 so
Q1=W1=P0V0lnV04V0=P0V0ln4=P0V0(2ln2). -
Isobaric Compression at P=P0/4 from 4V0 to V0:
Temperatures:
Initial: T2=RP0/4×4V0=RP0V0
Final: T3=RP0/4×V0=4RP0V0
ΔT=T3−T2=−4R3P0V0.
First law: Q2=ΔU+W2.
Work done:
W2=PΔV=4P0(V0−4V0)=−43P0V0.Change in internal energy:
ΔU=nCVΔT=−43P0V0RCV.Thus,
Q2=−43P0V0(RCV+1).Note: We will see that the contribution due to CV cancels with the next step.
-
Isochoric Heating at V0 from T3=4RP0V0 back to the initial temperature T1=RP0V0:
Since W3=0,
Q3=ΔU=nCVΔT=nCV(RP0V0−4RP0V0)=43P0V0RCV.
Adding the contributions:
Qtotal=Q1+Q2+Q3=P0V0(2ln2)−43P0V0(RCV+1)+43P0V0RCV.Notice that the terms with RCV cancel:
Qtotal=P0V0(2ln2)−43P0V0.Thus,
Qtotal=P0V0(2ln2−0.75).