Question
Question: Which of the following has correct numbering according IUPAC :...
Which of the following has correct numbering according IUPAC :
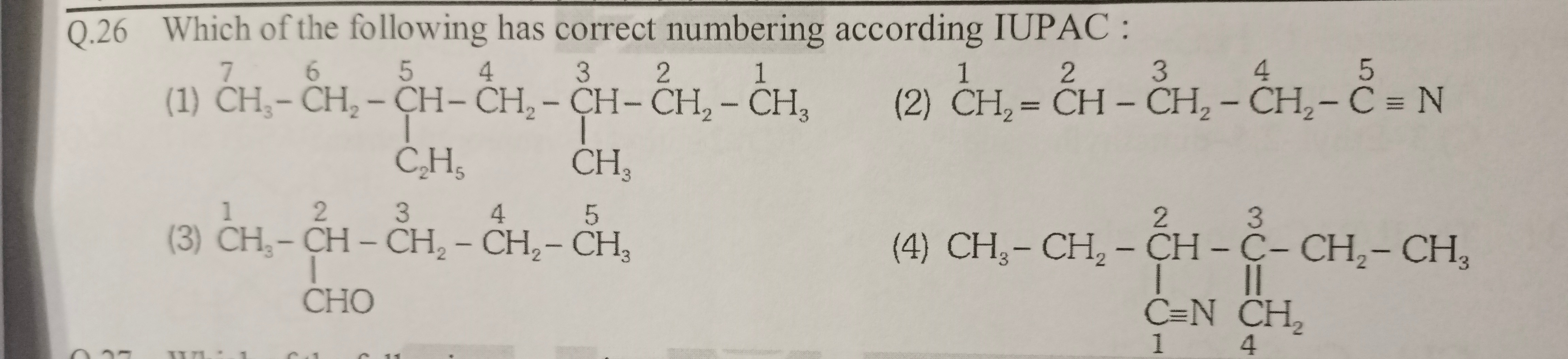
CH3−CH2−CH26−CH5−CH24−CH3−CH22−CH31
∣
C2H5
∣
CH3
CH21=CH2−CH23−CH24−C5≡N
CH31−CH2−CH23−CH24−CH35 ∣ CHO
CH3−CH2−CH2−C3−CH2−CH3 ∣∣∣ C≡NCH2 14
Option (1) has the correct IUPAC numbering.
Solution
Let's analyze each option based on IUPAC rules.
Option (1):
The structure is CH3−CH2−CH2−CH(C2H5)−CH2−CH(CH3)−CH2−CH3.
Expanding the ethyl group (C2H5) to CH2CH3:
CH3−CH2−CH2−CH(CH2CH3)−CH2−CH(CH3)−CH2−CH3
-
Identify the longest continuous carbon chain (parent chain):
- Straight chain: CH3−CH2−CH2−CH−CH2−CH−CH2−CH3 (8 carbons, octane).
- Chain including the ethyl group: Start from the CH3 of the ethyl group, go through the CH2 of the ethyl group, then to the CH it's attached to, and continue along the longest path. For example, CH3(ethyl)−CH2(ethyl)−CH−CH2−CH−CH2−CH2−CH3. This chain also has 8 carbons.
When two chains of equal length are possible, the one with the maximum number of substituents is chosen. Both chains (straight and the one incorporating ethyl) result in 2 substituents. In such cases, the chain that gives the lowest set of locants to the substituents is chosen.
-
Number the parent chain to give substituents the lowest possible locants: Let's consider the straight 8-carbon chain (octane).
- Numbering from right to left (as shown in the option):
CH3(8)−CH2(7)−CH2(6)−CH(5)−CH2(4)−CH(3)−CH2(2)−CH3(1)
The substituents are: methyl at C3 and ethyl at C5. The set of locants is (3, 5). - Numbering from left to right:
CH3(1)−CH2(2)−CH2(3)−CH(4)−CH2(5)−CH(6)−CH2(7)−CH3(8)
The substituents are: ethyl at C4 and methyl at C6. The set of locants is (4, 6).
Comparing the sets of locants, (3, 5) is lower than (4, 6). Therefore, numbering from right to left is correct for this parent chain. The numbering shown in option (1) matches this correct numbering.
The name would be 5-ethyl-3-methyloctane. - Numbering from right to left (as shown in the option):
Option (2):
CH21=CH2−CH23−CH24−C5≡N
This molecule contains a double bond and a nitrile group. According to IUPAC priority rules, the nitrile group (−C≡N) has higher priority than a double bond. The carbon of the nitrile group must be assigned the lowest possible number, which is C1.
The correct numbering should be:
CH25=CH4−CH23−CH22−C1≡N
The given numbering starts from the double bond end, making the nitrile carbon C5, which is incorrect.
Option (3):
CH31−CH2−CH23−CH24−CH35
∣
CHO
This molecule contains an aldehyde group (−CHO). The carbon of the aldehyde group must be part of the main chain and be assigned C1.
The longest chain containing the aldehyde group is:
CH3−CH2−CH2−CH(CH3)−CHO
The correct numbering should start from the aldehyde carbon:
CH35−CH24−CH23−CH2−CHO1
|
CH3
The given numbering starts from the methyl group on the left, making the aldehyde carbon C2, which is incorrect.
Option (4):
CH3−CH2−CH2−C3−CH2−CH3
∣∣∣
C≡NCH2
14
This molecule contains a nitrile group (−C≡N) and an alkene group (−C=CH2).
According to IUPAC priority rules, the nitrile group has higher priority than an alkene. The carbon of the nitrile group must be C1. The numbering should also ensure the double bond gets the lowest possible locant.
Based on the analysis, only option (1) has correct numbering according to IUPAC rules for the chosen parent chain.
