Question
Question: For the reaction : 2CO(g) + O2(g) → 2CO2(g) Following data is given : <figure/> The overall order ...
For the reaction : 2CO(g) + O2(g) → 2CO2(g)
Following data is given :
The overall order of reaction is -
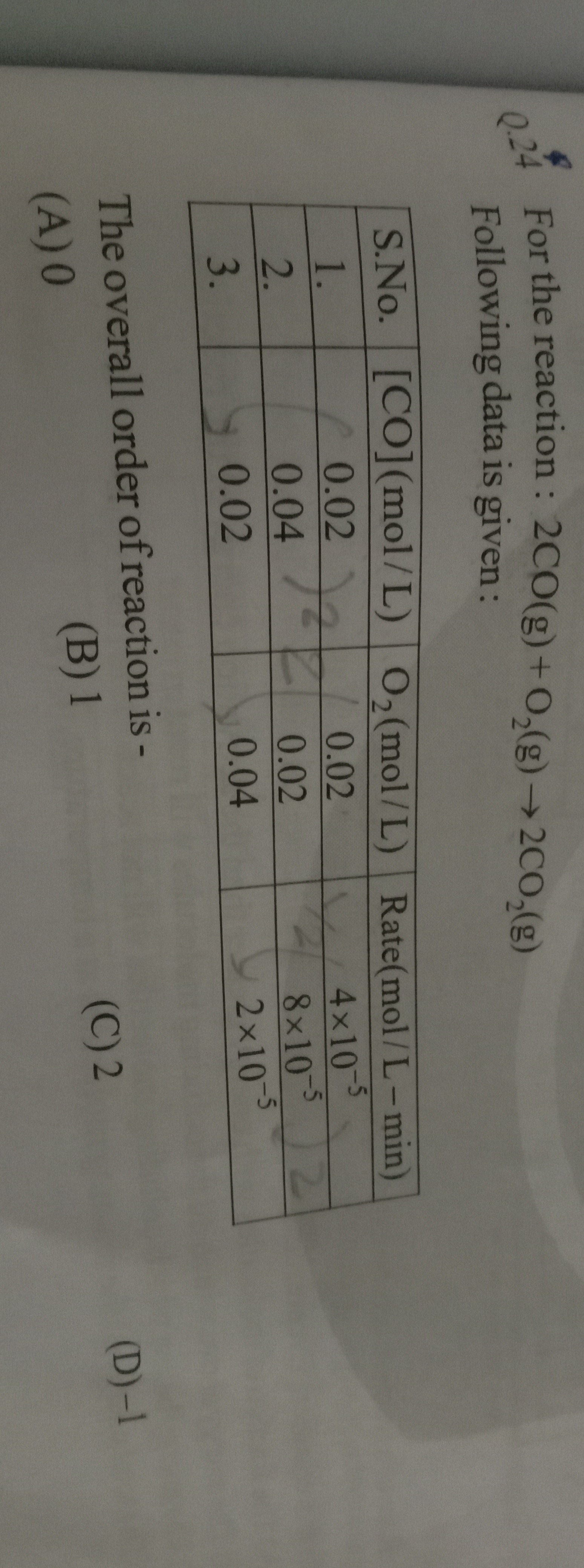
0
1
2
-1
0
Solution
The reaction is given as: 2CO(g)+O2(g)→2CO2(g)
The rate law for the reaction can be expressed as: Rate=k[CO]x[O2]y
where 'x' is the order of the reaction with respect to CO, 'y' is the order of the reaction with respect to O2, and 'k' is the rate constant. The overall order of the reaction is x+y.
We use the provided experimental data to determine the values of 'x' and 'y'.
1. Determine the order with respect to CO (x):
Compare experiments 1 and 2, where the concentration of O2 is kept constant.
From Experiment 1: 4×10−5=k[0.02]x[0.02]y (Equation 1)
From Experiment 2: 8×10−5=k[0.04]x[0.02]y (Equation 2)
Divide Equation 2 by Equation 1:
4×10−58×10−5=k[0.02]x[0.02]yk[0.04]x[0.02]y
2=(0.020.04)x
2=(2)x
Therefore, x=1.
The reaction is first order with respect to CO.
2. Determine the order with respect to O2 (y):
Compare experiments 1 and 3, where the concentration of CO is kept constant.
From Experiment 1: 4×10−5=k[0.02]x[0.02]y (Equation 1)
From Experiment 3: 2×10−5=k[0.02]x[0.04]y (Equation 3)
Divide Equation 3 by Equation 1:
4×10−52×10−5=k[0.02]x[0.02]yk[0.02]x[0.04]y
0.5=(0.020.04)y
21=(2)y
2−1=(2)y
Therefore, y=−1.
The reaction is minus first order with respect to O2.
3. Calculate the overall order of the reaction:
Overall order = x+y
Overall order = 1+(−1)
Overall order = 0
The overall order of the reaction is 0.
