Question
Question: Q2. Identify oxidizing and reducing agent in the following reactions: a) Fe(s) + Cu$^{2+}$(aq) → Fe...
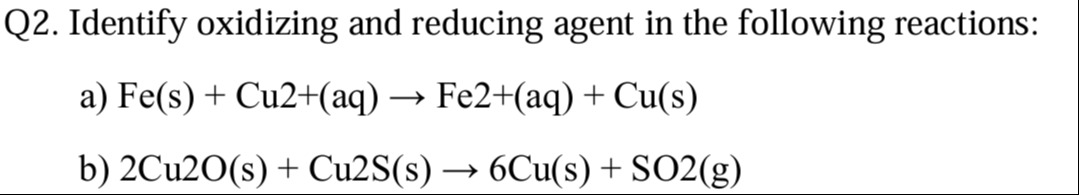
Q2. Identify oxidizing and reducing agent in the following reactions:
a) Fe(s) + Cu2+(aq) → Fe2+(aq) + Cu(s)
b) 2Cu2O(s) + Cu2S(s) → 6Cu(s) + SO2(g)
Answer
a) Oxidizing agent: Cu2+; Reducing agent: Fe b) Oxidizing agent: Cu2O; Reducing agent: Cu2S
Explanation
Solution
(a) Fe is oxidized from 0 to +2 (reducing agent) and Cu2+ is reduced to 0 (oxidizing agent).
(b) In Cu2S, S is oxidized from -2 to +4 (making Cu2S the reducing agent) while Cu+ ions (in Cu2O) are reduced to 0 (making Cu2O the oxidizing agent).
