Question
Question: Enzymes convert glucose ($M$ = 180.2) to ethanol ($M$ = 46.1) according to the equation. $C_6H_{12}...
Enzymes convert glucose (M = 180.2) to ethanol (M = 46.1) according to the equation.
C6H12O6→2C2H5OH+2CO2. What is the maximum mass of ethanol that can be made from 15.5 kg of glucose?
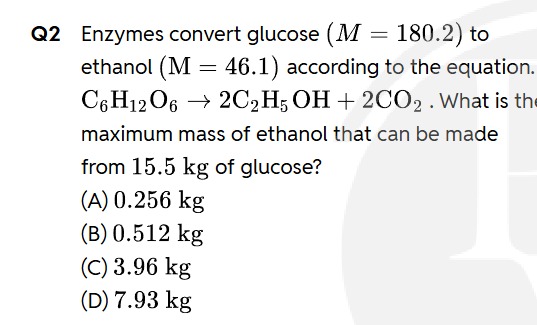
0.256 kg
0.512 kg
3.96 kg
7.93 kg
7.93 kg
Solution
The balanced chemical equation is: C6H12O6→2C2H5OH+2CO2
-
Convert the mass of glucose from kg to g:
15.5 kg×1000 g/kg=15500 g
-
Calculate the moles of glucose:
Moles of glucose=180.2 g/mol15500 g=86.01 mol
-
Determine the moles of ethanol produced:
From the balanced equation, 1 mole of glucose produces 2 moles of ethanol.
Moles of ethanol=2×86.01 mol=172.02 mol
-
Calculate the mass of ethanol:
Mass of ethanol=172.02 mol×46.1 g/mol=7930.12 g
-
Convert the mass of ethanol from g to kg:
Mass of ethanol=1000 g/kg7930.12 g=7.93 kg
Therefore, the maximum mass of ethanol that can be made from 15.5 kg of glucose is approximately 7.93 kg.
