Question
Question: Write down IUPAC of following compounds. i) $CH_3-CH_2-\begin{gathered}CH\\|\\C_2H_5\end{gathered}-...
Write down IUPAC of following compounds.
i) CH3−CH2−CH∣C2H5−CH2−CH∣CH3−CH3
ii) CH3∣CH3−C−CH3∣C2H5
iii) CH3∣CH3−C−CH3∣CH3
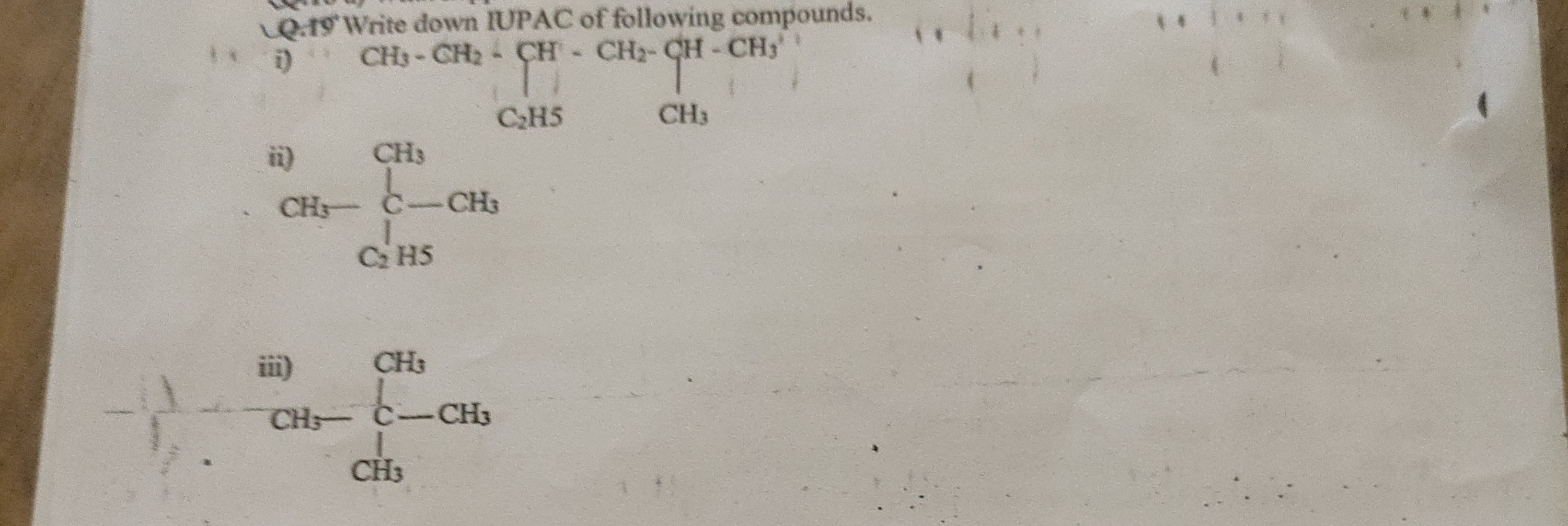
(i) 4-ethyl-2-methylhexane
(ii) 2,2-dimethylbutane
(iii) 2,2-dimethylpropane
Solution
Solution:
We have to name the compounds by first selecting the longest continuous carbon chain and then assigning locants so that the substituents get the lowest numbers.
(i)
Structure:
CH₃–CH₂–[CH(CH₂CH₃)]–CH₂–[CH(CH₃)]–CH₃
– Counting the main chain from left to right gives a six‐carbon chain (hexane).
– There is an ethyl (–C₂H₅) substituent on what appears to be C3 and a methyl (–CH₃) on C5.
– However, when numbered from the other end the substituent locants become 2 and 4 (because then the ethyl group falls on C4 and one methyl on C2).
– According to the lowest set rule the “right‐to‐left” numbering (locants 2 and 4) is preferred.
– Thus the main chain is hexane with a methyl at C2 and an ethyl at C4.
– In the final name the substituents are listed in alphabetical order (“ethyl” before “methyl”).
Name (i):
4‑ethyl‑2‑methylhexane
(ii)
Structure (drawing):
CH₃ │ CH₃– C – CH₃ │ C₂H₅
Here the central carbon is attached to four groups: three methyls and one ethyl.
– To choose the longest chain we find the maximum continuous carbon chain by “bridging” through the central carbon.
– The longest path is obtained by taking the two “longest” branches: the ethyl group (2 carbons) and one of the methyl groups (1 carbon), connected via the central carbon. This gives a chain of 1 + 1 + 2 = 4 carbons (butane).
– When the chosen chain is drawn as
CH₃–[central C]–CH₂–CH₃,
the central carbon (which becomes C2) already carries a substituent (the methyl group that is part of the chain) and the two remaining methyl groups become substituents on C2.
– Hence the parent is butane with two extra methyl groups at C2.
Name (ii):
2,2‑dimethylbutane
(iii)
Structure (drawing):
CH₃ │ CH₃– C – CH₃ │ CH₃
The central carbon is now attached to four methyl groups.
– The longest chain can be chosen by taking any one of the peripheral CH₃ groups, the central carbon, and another CH₃ group attached to it; this gives a three‐carbon chain (propane).
– The central carbon, being C2, then carries the two remaining CH₃ groups as substituents.
Name (iii):
2,2‑dimethylpropane
Core Explanation (minimal):
-
(i): Longest chain is hexane. Right-to-left numbering gives substituent positions 2 (methyl) and 4 (ethyl). Listing substituents alphabetically, the name is
4‑ethyl‑2‑methylhexane. -
(ii): The structure has a quaternary carbon with three CH₃ and one C₂H₅. The longest chain is the 4‑carbon chain (butane) passing through the quaternary carbon. The two extra CH₃ groups attached at C2 give
2,2‑dimethylbutane. -
(iii): With four CH₃ groups on the central carbon, the longest chain is propane; the two extra CH₃ groups at C2 give
2,2‑dimethylpropane.
