Question
Question: Major product of this reaction is ...
Major product of this reaction is
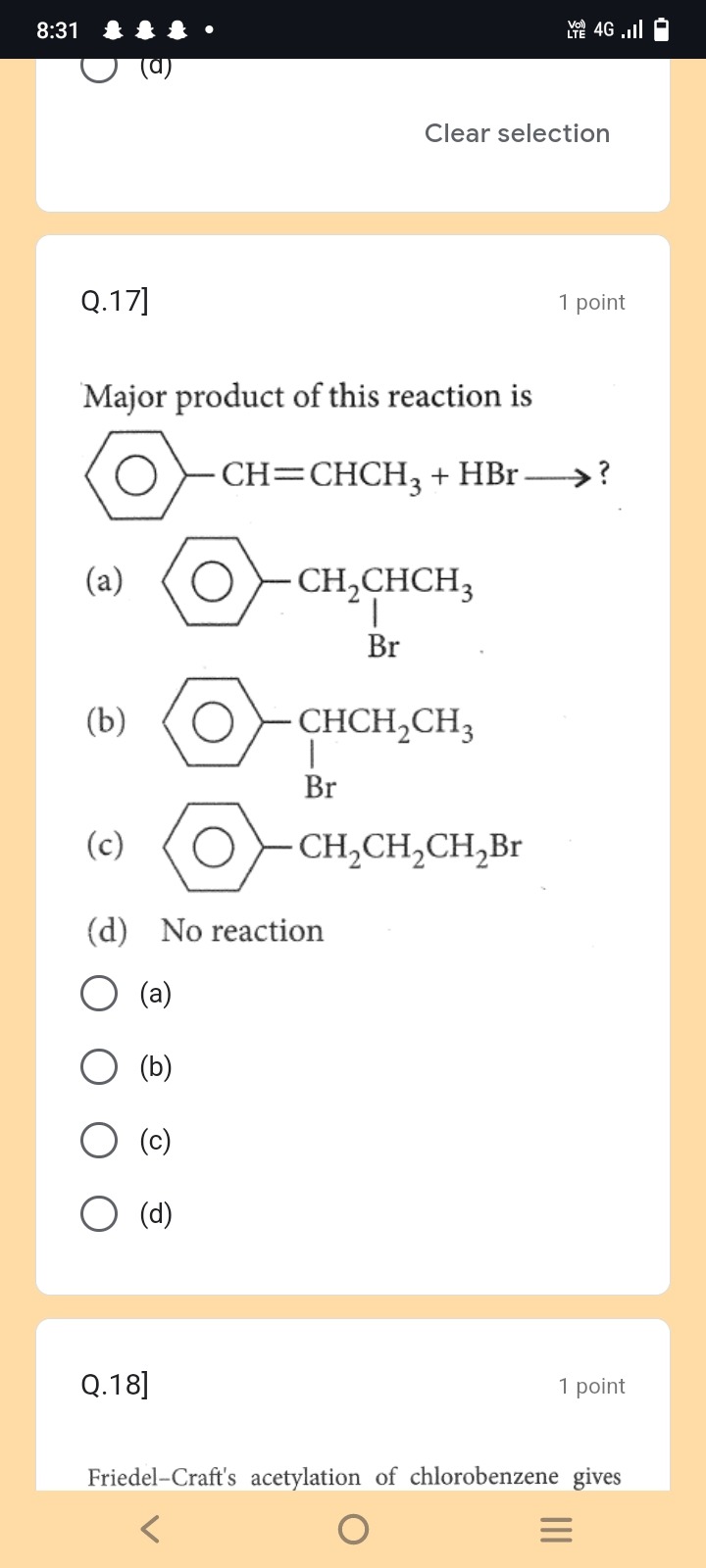
A
B
C
D
No reaction
Answer
Option (b)
Explanation
Solution
-
The substrate is Ph–CH=CHCH₃.
-
On treatment with HBr, the proton adds in a Markovnikov fashion.
-
The proton adds to the terminal carbon so that the carbocation forms at the benzylic position (stabilized by resonance).
-
Bromide then attacks the benzylic carbocation giving Ph–CH(Br)–CH₂CH₃.
-
This corresponds to option (b).
