Question
Question: The given reaction will produce which of the following as the major product?...
The given reaction will produce which of the following as the major product?
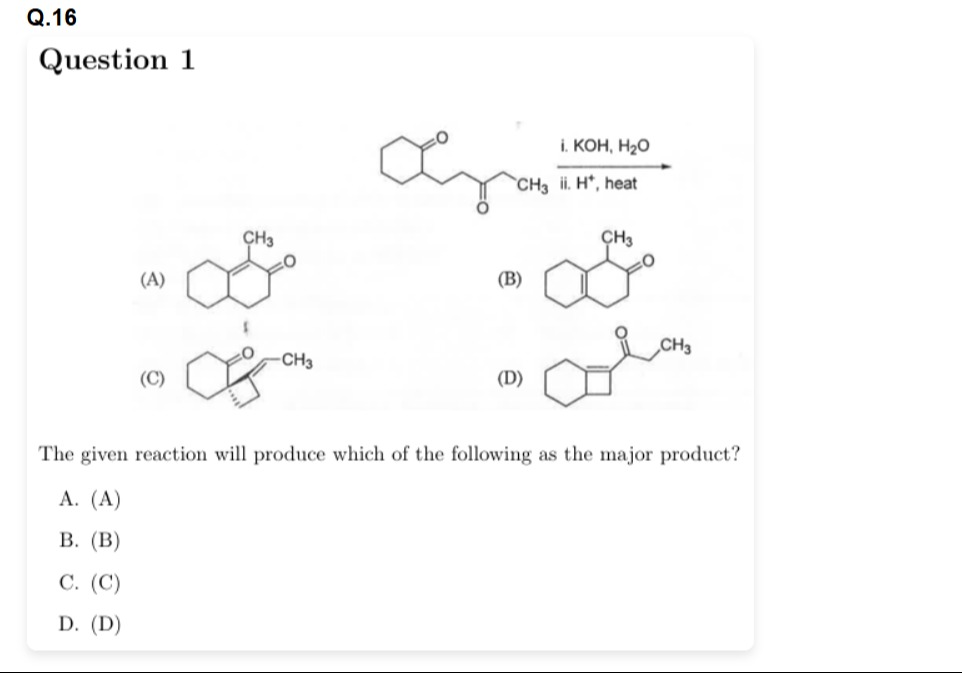
(A)
(B)
(C)
(D)
A
Solution
The reaction is an intramolecular aldol condensation followed by dehydration. The starting material is 1-(2-oxocyclohexyl)butan-3-one.
In basic conditions (KOH, H2O), an enolate is formed. There are several possible alpha hydrogens. The most acidic alpha hydrogens are typically those between two carbonyl groups, but in this case, the methylene group between the two carbonyls is part of the chain connecting the two carbonyls in an unfavorable position for forming a stable ring.
Intramolecular aldol condensation is favored when it leads to the formation of 5- or 6-membered rings.
Let's consider the formation of a 6-membered ring by attack on the cyclohexanone carbonyl. This requires the enolate to be formed at the methyl carbon of the acetyl group.
Let's consider the formation of a 6-membered ring by intramolecular aldol condensation where the enolate is formed on the cyclohexanone ring and attacks the side chain carbonyl.
Let's consider the formation of a 6-membered ring where the enolate is formed on the side chain and attacks the cyclohexanone carbonyl.
Let's re-examine the options, particularly option A. It is a bicyclo[4.4.0]dec-1-en-3-one with a methyl group. Let's try to construct this from the starting material.
