Question
Question: An electrochemical cell is fueled by the combustion of butane at 1 bar and 298 K. Its cell potential...
An electrochemical cell is fueled by the combustion of butane at 1 bar and 298 K. Its cell potential is FX×103 volts, where F is the Faraday constant. The value of X is ____.
Use: Standard Gibbs energies of formation at 298 K are: ΔfGCO2o=−394 kJ mol−1; ΔfGwatero=−237 kJ mol−1; ΔfGbutaneo=−18 kJ mol−1
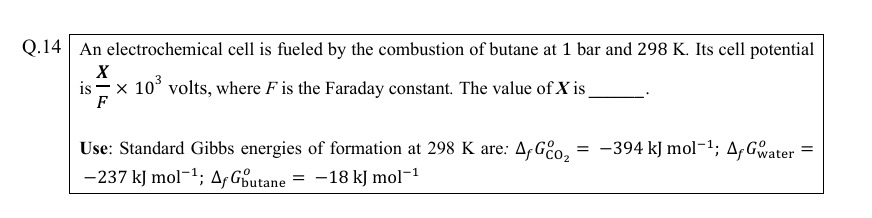
105.5
Solution
The balanced chemical equation for the combustion of butane is:
C4H10(g)+6.5O2(g)→4CO2(g)+5H2O(l)
We are given the standard Gibbs energies of formation at 298 K:
ΔfGCO2o=−394 kJ mol−1
ΔfGwater(l)o=−237 kJ mol−1
ΔfGbutane(g)o=−18 kJ mol−1
The standard Gibbs energy of formation of O2(g) in its standard state is 0 kJ mol−1.
The standard Gibbs free energy change for the reaction (ΔrGo) is calculated as:
ΔrGo=∑(stoichiometric coefficient×ΔfGproductso)−∑(stoichiometric coefficient×ΔfGreactantso)
ΔrGo=[4×ΔfGCO2o+5×ΔfGH2O(l)o]−[1×ΔfGC4H10(g)o+6.5×ΔfGO2(g)o]
ΔrGo=[4×(−394 kJ/mol)+5×(−237 kJ/mol)]−[1×(−18 kJ/mol)+6.5×(0 kJ/mol)]
ΔrGo=[−1576 kJ/mol−1185 kJ/mol]−[−18 kJ/mol]
ΔrGo=−2761 kJ/mol+18 kJ/mol
ΔrGo=−2743 kJ/mol
Convert ΔrGo to Joules per mole:
ΔrGo=−2743×103 J/mol
The relation between standard Gibbs free energy change and standard cell potential (Eo) is:
ΔrGo=−nFEo
where n is the number of moles of electrons transferred in the reaction, and F is the Faraday constant.
To find n, we examine the changes in oxidation states.
In C4H10, the oxidation state of C is -2.5. The total oxidation state for 4 carbons is 4×(−2.5)=−10.
In CO2, the oxidation state of C is +4. The total oxidation state for 4 carbons is 4×(+4)=+16.
The change in total oxidation state of carbon is +16−(−10)=+26. This corresponds to the loss of 26 electrons.
In O2, the oxidation state of O is 0.
In CO2 and H2O, the oxidation state of O is -2.
There are 6.5 moles of O2, which contain 6.5×2=13 moles of oxygen atoms.
Each oxygen atom changes its oxidation state from 0 to -2, which is a gain of 2 electrons.
The total number of electrons gained by oxygen is 13×2=26.
So, the number of electrons transferred is n=26.
Now, we can calculate Eo:
Eo=nF−ΔrGo
Eo=26×F C/mol−(−2743×103 J/mol)
Eo=26F2743×103 V
The cell potential is given in the form FX×103 volts.
Comparing this with our calculated Eo:
FX×103=262743×F103
X=262743
Calculate the value of X:
X=262743=105.5
The value of X is 105.5.
