Question
Question: Two elements **A** and **B** combine to form compound **X** and **Y**. For the fixed mass of **A**, ...
Two elements A and B combine to form compound X and Y. For the fixed mass of A, masses of B combined for the compounds X and Y are in 3 : 7 ratio. If in compound X, 4 g of A combines with 12 g B, then in compound Y, 8 g of A will combine with...................g of B.
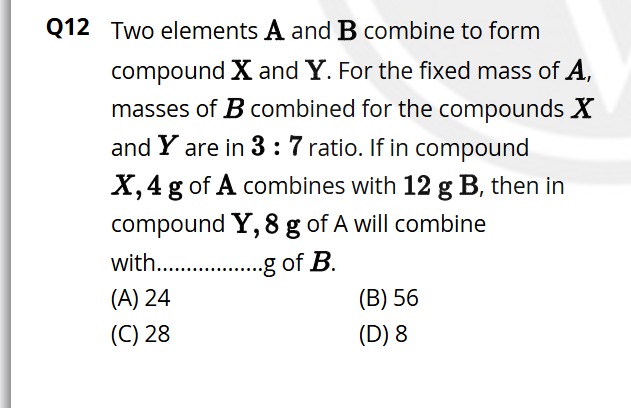
24
56
28
8
56
Solution
Let the two compounds formed by elements A and B be X and Y.
In compound X, 4 g of A combines with 12 g of B. The ratio of mass of B to mass of A in compound X is Mass of AMass of B=4 g12 g=3.
The problem states that for a fixed mass of A, the masses of B combined for compounds X and Y are in the ratio 3 : 7. Let's consider a fixed mass of A, say 4 g (as given for compound X). For this fixed mass of A (4 g), the mass of B in compound X is 12 g. Let the mass of B that combines with 4 g of A in compound Y be mY. According to the given ratio:
Mass of B in Y (for 4g A)Mass of B in X (for 4g A)=73
mY12 g=73
mY=312 g×7=4 g×7=28 g. So, in compound Y, 4 g of A combines with 28 g of B.
Now, we need to find the mass of B that combines with 8 g of A in compound Y. According to the Law of Definite Proportions, the ratio of masses of elements in a chemical compound is fixed. In compound Y, the ratio of mass of B to mass of A is constant:
Mass of AMass of B=4 g28 g=7.
If 8 g of A combines with mB′ g of B in compound Y, then:
8 gmB′=7
mB′=7×8 g=56 g.
Therefore, in compound Y, 8 g of A will combine with 56 g of B.
