Question
Question: Consider a reaction $A + R \rightarrow Product$. The rate of this reaction is measured to be $k[A][R...
Consider a reaction A+R→Product. The rate of this reaction is measured to be k[A][R]. At the start of the reaction, the concentration of R, [R]0, is 10-times the concentration of A, [A]0. The reaction can be considered to be a pseudo first order reaction with assumption that k[R]=k′ is constant. Due to this assumption, the relative error (in %) in the rate when this reaction is 40 % complete, is ______.
[k and k' represent corresponding rate constants]
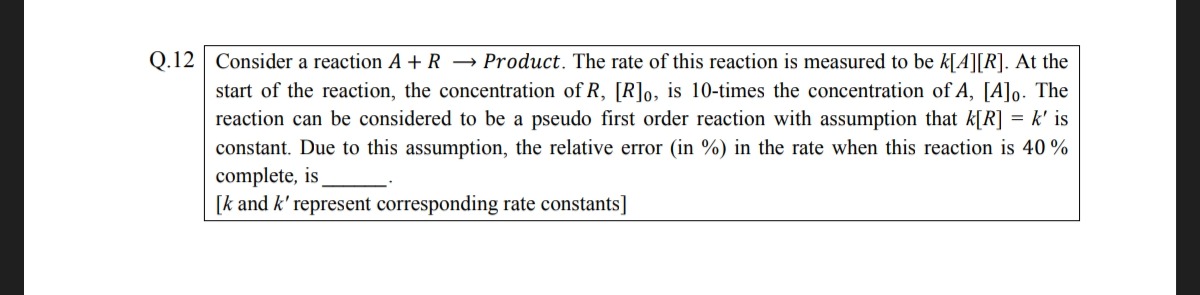
4.166
Solution
Let the reaction be A+R→Product. The rate of the reaction is given by rate=k[A][R]. At the start of the reaction, [R]0=10[A]0.
The reaction is considered to be a pseudo first order reaction with the assumption that k[R]=k′ is constant. This assumption is typically made by assuming that the concentration of the excess reactant R remains approximately constant at its initial value [R]0. So, the pseudo first order rate constant is k′=k[R]0. The pseudo first order rate expression is ratepseudo=k′[A]=k[R]0[A].
The actual rate expression is rateactual=k[A][R].
The reaction is 40% complete. This means that 40% of the initial concentration of A has reacted. Let [A]0 be the initial concentration of A. The concentration of A at 40% completion is [A]t=[A]0−0.40[A]0=0.60[A]0.
According to the stoichiometry of the reaction A+R→Product, when 1 mole of A reacts, 1 mole of R also reacts. The amount of A reacted is 0.40[A]0. So, the amount of R reacted is also 0.40[A]0. The initial concentration of R is [R]0. The concentration of R at 40% completion is [R]t=[R]0−0.40[A]0. Given [R]0=10[A]0, we have [R]t=10[A]0−0.40[A]0=(10−0.40)[A]0=9.60[A]0.
Now, let's calculate the actual rate and the pseudo rate at 40% completion.
The actual rate at 40% completion is rateactual=k[A]t[R]t=k(0.60[A]0)(9.60[A]0)=k(0.60×9.60)[A]02=5.76k[A]02.
The pseudo first order rate at 40% completion is ratepseudo=k[R]0[A]t=k(10[A]0)(0.60[A]0)=k(10×0.60)[A]02=6.00k[A]02.
The relative error in the rate is the difference between the pseudo rate (approximate value) and the actual rate (true value), divided by the actual rate (true value).
RelativeError=rateactual∣ratepseudo−rateactual∣.
RelativeError=5.76k[A]02∣6.00k[A]02−5.76k[A]02∣=5.76k[A]02∣6.00−5.76∣k[A]02=5.760.24.
To simplify the fraction 5.760.24:
5.760.24=57624.
We can divide both the numerator and the denominator by 24.
24÷24=1.
576÷24=24.
So, the relative error is 241.
The question asks for the relative error in percentage (%).
PercentageRelativeError=RelativeError×100%.
PercentageRelativeError=241×100%=24100%.
24100=1250=625.
PercentageRelativeError=625%.
As a decimal, 625=4.1666...
The question asks for the relative error (in %), which suggests a numerical value is expected. The value is 625.
