Question
Question: In the relation $P = \frac{\alpha}{\beta}e^{\frac{-\alpha z}{K\theta}}P$ is pressure, $Z$ is the dis...
In the relation P=βαeKθ−αzP is pressure, Z is the distance, K is Boltzmann's constant and θ is the temperature. The dimensional formula of α will be:
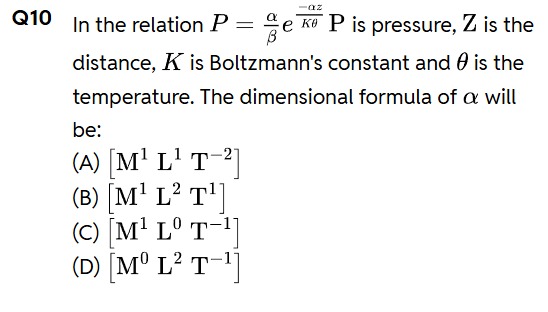
[M1L1T−2]
[M1L2T1]
[M1L0T−1]
[M0L2T−1]
[M1L1T−2]
Solution
The given relation is P=βαeKθ−αz. Here, P is pressure, Z is the distance, K is Boltzmann's constant and θ is the temperature.
For the equation to be dimensionally consistent, the exponent of the exponential term must be dimensionless. So, the dimension of Kθ−αz is [M0L0T0]. This means the dimension of Kθαz is also [M0L0T0].
[Kθαz]=[M0L0T0]
[α][z][Kθ]−1=[M0L0T0]
[α][z]=[Kθ]
We need to find the dimensions of [z] and [Kθ].
Z is distance, so [z]=[L].
K is Boltzmann's constant and θ is temperature. The product Kθ has the dimensions of energy. This can be seen from the ideal gas law PV=NKT, where PV has dimensions of energy ([P]=[ML−1T−2], [V]=[L3], so [PV]=[ML2T−2]), N is the number of molecules (dimensionless), so [KT]=[PV]=[ML2T−2]. Since the temperature is denoted by θ, [Kθ]=[ML2T−2].
Substitute these dimensions into the equation [α][z]=[Kθ]:
[α][L]=[ML2T−2]
Now, solve for the dimension of α:
[α]=[L][ML2T−2]
[α]=[ML2−1T−2]
[α]=[ML1T−2]
The dimensional formula of α is [M1L1T−2].
Therefore, the correct answer is (A).
