Question
Question: Q.10 $\Delta$ G° of the cell reaction $AgCl(s) + \frac{1}{2}H_2(g) \rightarrow Ag(s) + H^+ + Cl^-$ i...
Q.10 Δ G° of the cell reaction AgCl(s)+21H2(g)→Ag(s)+H++Cl− is -21.52 KJ Δ G° of 2AgCl(s)+H2(g)→2Ag(s)+2H++2Cl− is:
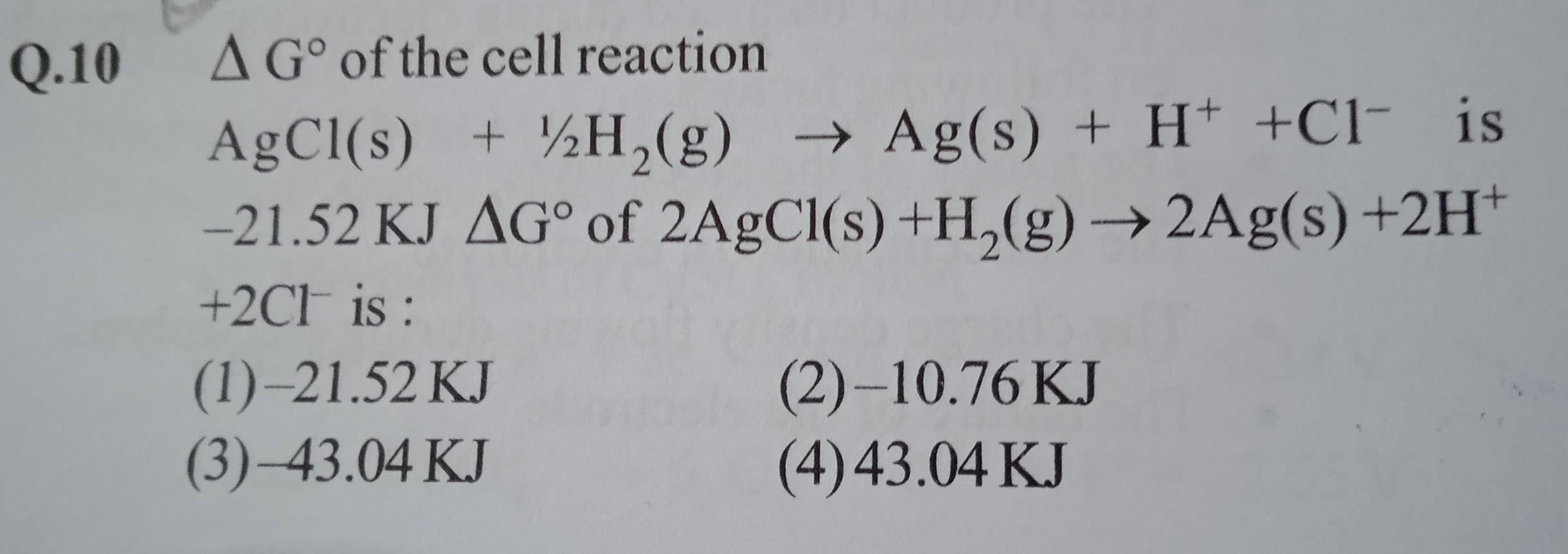
-21.52 KJ
-43.04 KJ
-10.76 KJ
43.04 KJ
-43.04 KJ
Solution
The Gibbs free energy change (ΔG∘) is an extensive property, meaning its value depends on the amount of substance involved in the reaction. If a chemical reaction is multiplied by a stoichiometric factor, the corresponding ΔG∘ value is also multiplied by the same factor.
The given reaction is:
AgCl(s)+21H2(g)→Ag(s)+H++Cl−
For this reaction, ΔG1∘=−21.52 KJ.
The reaction for which ΔG∘ is to be found is:
2AgCl(s)+H2(g)→2Ag(s)+2H++2Cl−
Comparing the two reactions, it is clear that the second reaction is exactly twice the first reaction. If we multiply the first reaction by 2:
2×(AgCl(s)+21H2(g)→Ag(s)+H++Cl−)
This gives:
2AgCl(s)+H2(g)→2Ag(s)+2H++2Cl−
Since the second reaction is obtained by multiplying the first reaction by a factor of 2, the ΔG∘ for the second reaction (ΔG2∘) will be twice the ΔG∘ of the first reaction (ΔG1∘).
ΔG2∘=2×ΔG1∘
ΔG2∘=2×(−21.52 KJ)
ΔG2∘=−43.04 KJ
