Question
Question: $\Delta$ G° of the cell reaction AgCl(s) + $\frac{1}{2}$H$_2$(g) $\rightarrow$ Ag(s) + H$^+$ +Cl$^-$...
Δ G° of the cell reaction AgCl(s) + 21H2(g) → Ag(s) + H+ +Cl− is -21.52 KJ ΔG° of 2AgCl(s) +H2(g) → 2Ag(s) +2H+ +2Cl− is :
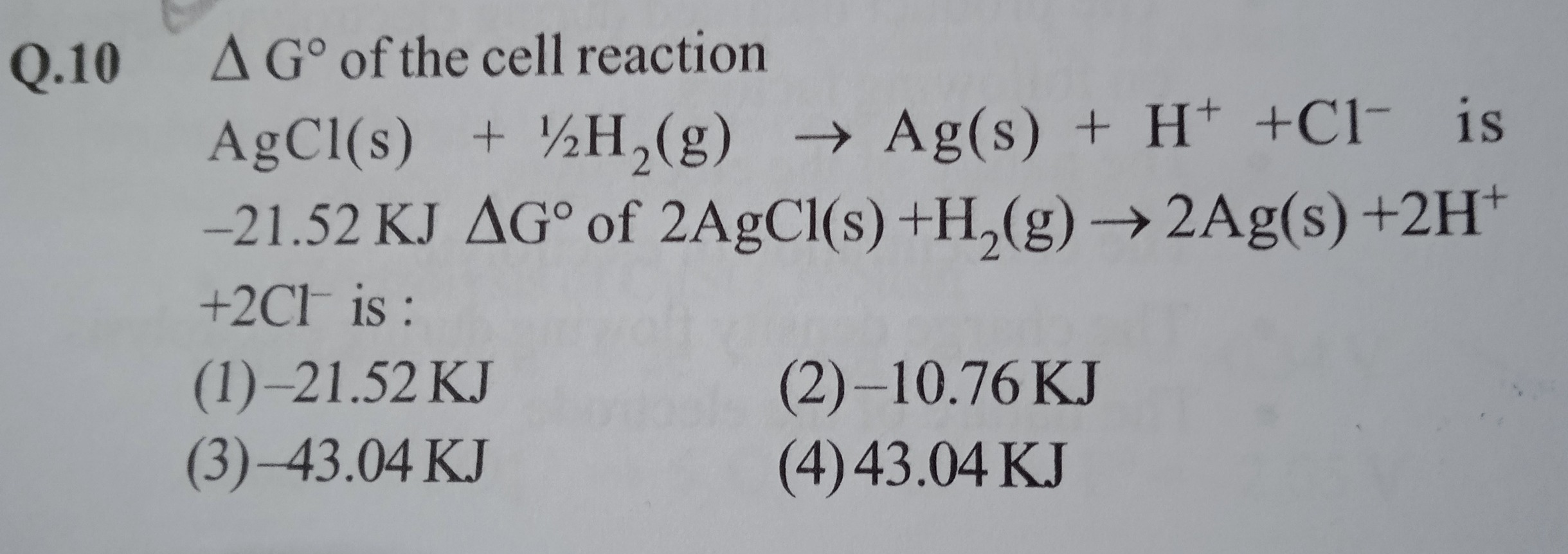
-21.52 KJ
-10.76 KJ
-43.04 KJ
43.04 KJ
-43.04 KJ
Solution
The given reaction is: AgCl(s) + 21H2(g) → Ag(s) + H+ +Cl−
For this reaction, ΔG° = -21.52 KJ.
The reaction for which ΔG° is to be calculated is: 2AgCl(s) + H2(g) → 2Ag(s) + 2H+ + 2Cl−
Upon comparing the two reactions, it is evident that the second reaction is exactly twice the first reaction. Since Gibbs free energy (ΔG°) is an extensive property, its value is directly proportional to the stoichiometric coefficients of the reaction. If a reaction is multiplied by a factor 'n', its ΔG° value is also multiplied by 'n'.
In this case, the second reaction is 2 times the first reaction. Therefore, ΔG° for the second reaction = 2 × (ΔG° for the first reaction)
ΔG° = 2 × (-21.52 KJ)
ΔG° = -43.04 KJ
