Question
Question: Q.1 $Zn | Zn^{2+}_{(ag)} || Cu^{2+}_{(ag)} | Cu$ Eº for the cell is 1.10 V at 25°C. The equilibrium ...
Q.1 Zn∣Zn(ag)2+∣∣Cu(ag)2+∣Cu Eº for the cell is 1.10 V at 25°C. The equilibrium constant for the cell reaction is of the order of
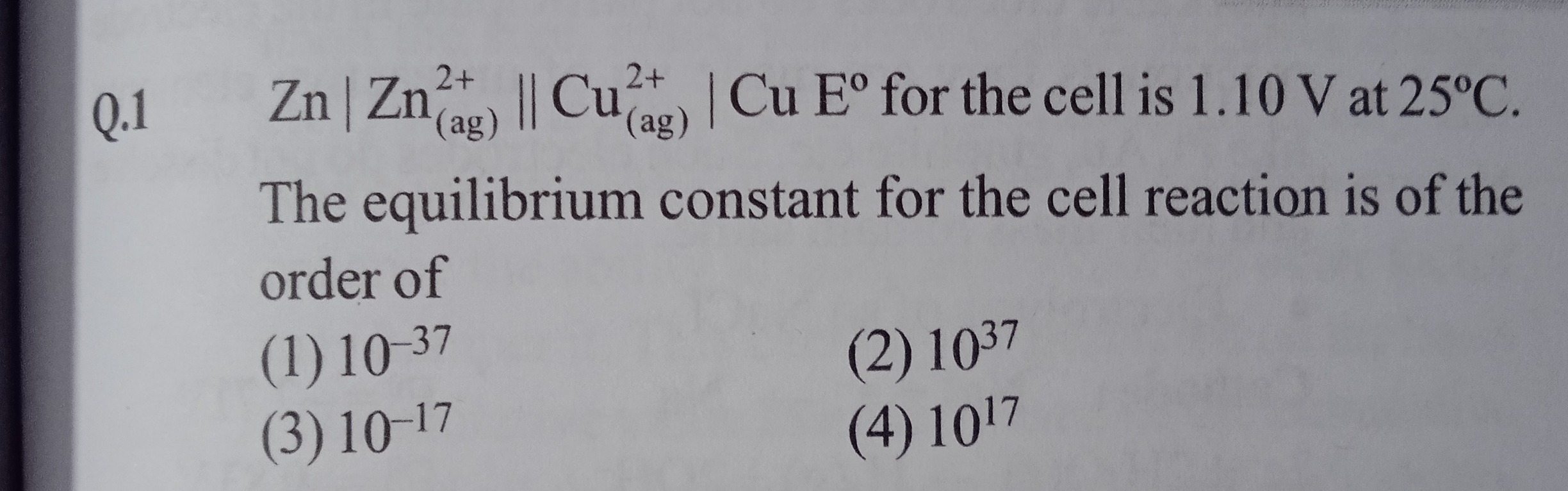
10−37
1037
10−17
1017
1037
Solution
The electrochemical cell given is Zn∣Zn(ag)2+∣∣Cu(ag)2+∣Cu. The standard cell potential (Ecell∘) is 1.10 V at 25°C.
The cell reaction can be written as:
Anode (oxidation): Zn(s)→Zn2+(aq)+2e−
Cathode (reduction): Cu2+(aq)+2e−→Cu(s)
Overall cell reaction: Zn(s)+Cu2+(aq)⇌Zn2+(aq)+Cu(s)
From the balanced cell reaction, the number of electrons transferred (n) is 2.
The relationship between the standard cell potential (Ecell∘) and the equilibrium constant (K) at 25°C is given by the formula: log10K=0.0592VnEcell∘
Given: Ecell∘=1.10V n=2
Substitute these values into the formula: log10K=0.05922×1.10 log10K=0.05922.20
Now, perform the division: log10K≈37.16
To find K, we take the antilog: K=1037.16
The question asks for the order of the equilibrium constant. Since K=1037.16, its order of magnitude is 1037.
Comparing this with the given options:
(1) 10−37
(2) 1037
(3) 10−17
(4) 1017
The calculated order matches option (2).
