Question
Question: In the given pair of alcohol, in which pair second alcohol is more reactive than first towards hydro...
In the given pair of alcohol, in which pair second alcohol is more reactive than first towards hydrogen bromide?
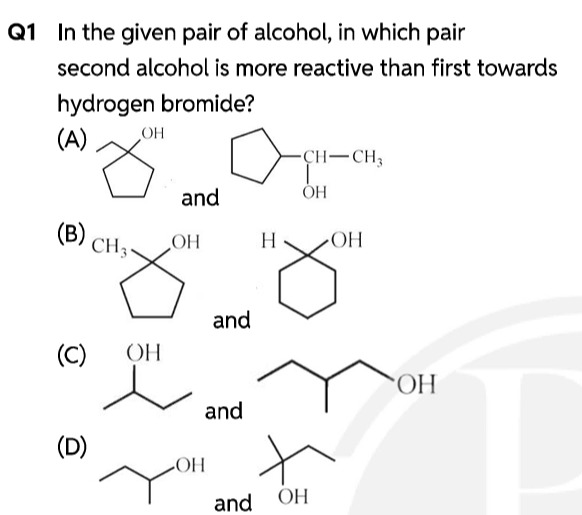
A
and
B
and
C
and
D
and
Answer
D
Explanation
Solution
The reactivity of alcohols towards hydrogen bromide (HBr) is determined by the stability of the carbocation intermediate formed during the reaction, which follows the order: Tertiary (3°) > Secondary (2°) > Primary (1°).
In option (D), the first alcohol (Butan-2-ol) is a secondary alcohol, and the second alcohol (2-methylbutan-2-ol) is a tertiary alcohol. Since tertiary alcohols are more reactive than secondary alcohols, the second alcohol is more reactive than the first.
