Question
Question: X is-...
X is-
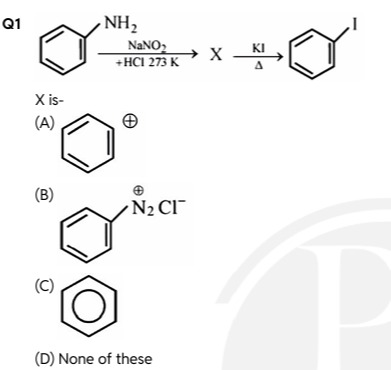
A
B
C
D
None of these
Answer
B
Explanation
Solution
The given reaction sequence involves two steps.
Step 1: Formation of X
The starting material, aniline (C₆H₅NH₂), reacts with sodium nitrite (NaNO₂) and hydrochloric acid (HCl) at a low temperature (273 K). This is diazotization, where an aromatic primary amine converts into an aromatic diazonium salt.
The reaction is:
C6H5NH2+NaNO2+2HCl273 KC6H5N2+Cl−+NaCl+2H2O
Compound X is benzenediazonium chloride.
Step 2: Reaction of X to form Iodobenzene
Compound X (benzenediazonium chloride) reacts with potassium iodide (KI) upon heating (Δ) to form iodobenzene (C₆H₅I).
C6H5N2+Cl−+KIΔC6H5I+N2+KCl
This confirms X is benzenediazonium chloride.
