Question
Question: w.o.f have $\Delta_o > P.E$? (SFL)...
w.o.f have Δo>P.E? (SFL)
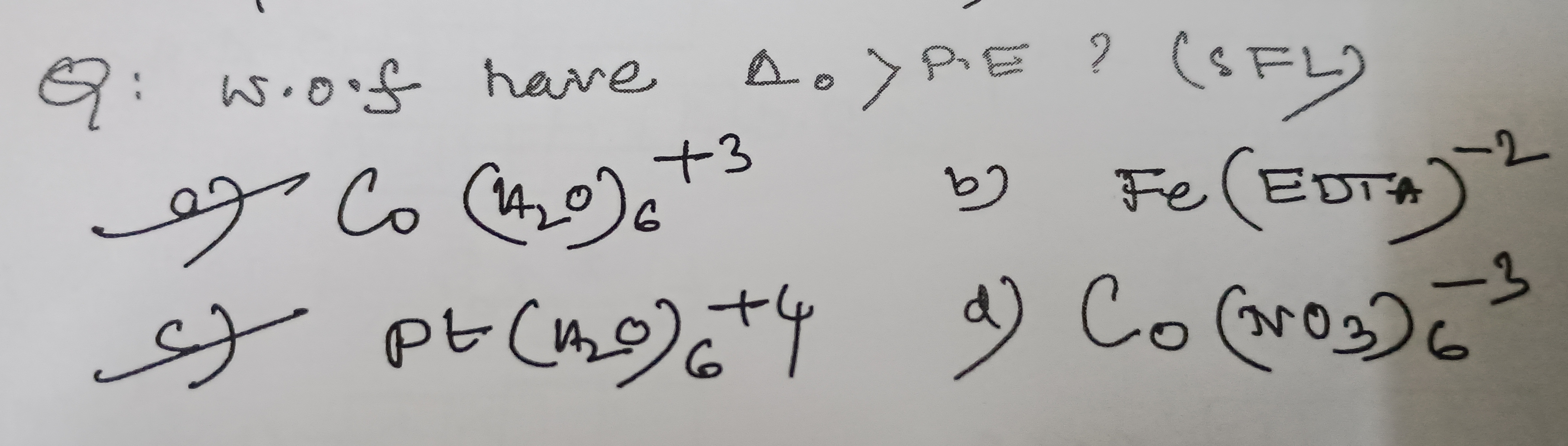
A
Co(H_2O)_6^{+3}
B
Fe(EDTA)^{-2}
C
Pt(H_2O)_6^{+4}
D
Co(NO_3)_6^{-3}
Answer
a, b, c
Explanation
Solution
The condition Δo>P.E implies a low spin complex. A low spin complex occurs when the crystal field splitting energy (Δo) is greater than the pairing energy (P.E.), meaning it is energetically more favorable for electrons to occupy the lower energy t2g orbitals before pairing up.
-
a) Co(H2O)6+3:
- Central metal ion: Cobalt (Co+3)
- d-electron configuration: 3d6
- Ligand: H2O (Water) is a weak field ligand.
- Analysis: For Co+3 (d6) with a weak field ligand, while H2O is weak, Co+3 has a strong tendency to form low spin complexes. The Δo for this complex is approximately 22000cm−1, and the pairing energy (P.E.) is around 17000cm−1. Thus, Δo>P.E.
-
b) Fe(EDTA)−2:
- Central metal ion: Iron (Fe+2)
- d-electron configuration: 3d6
- Ligand: EDTA (ethylenediaminetetraacetate) is a strong field ligand, especially due to its chelating nature.
- Analysis: With a strong field ligand like EDTA, the crystal field splitting energy (Δo) is significantly large, overcoming the pairing energy (P.E.). For Fe+2, P.E. is around 15000cm−1. A strong ligand ensures Δo>P.E.
-
c) Pt(H2O)6+4:
- Central metal ion: Platinum (Pt+4)
- d-electron configuration: 5d6
- Ligand: H2O (Water) is a weak field ligand.
- Analysis: Platinum is a 3rd-row transition metal. For 2nd and 3rd-row transition metals, Δo is significantly larger than for 1st-row metals. Even with a weak field ligand like H2O, the Δo for Pt+4 complexes is very large due to the metal's position in the periodic table and its high oxidation state. This ensures that Δo>P.E.
-
d) Co(NO3)6−3:
- Central metal ion: Cobalt (Co+3)
- d-electron configuration: 3d6
- Ligand: NO3− (Nitrate) is a weak field ligand.
- Analysis: For a d6 ion with a weak field ligand like NO3−, the crystal field splitting energy (Δo) is typically smaller than the pairing energy (P.E.). This leads to a high spin configuration, where Δo<P.E.
Therefore, complexes a), b), and c) satisfy the condition Δo>P.E. The correct options are a, b, and c.
