Question
Question: w.o.f have $\Delta_o > P.E$?...
w.o.f have Δo>P.E?
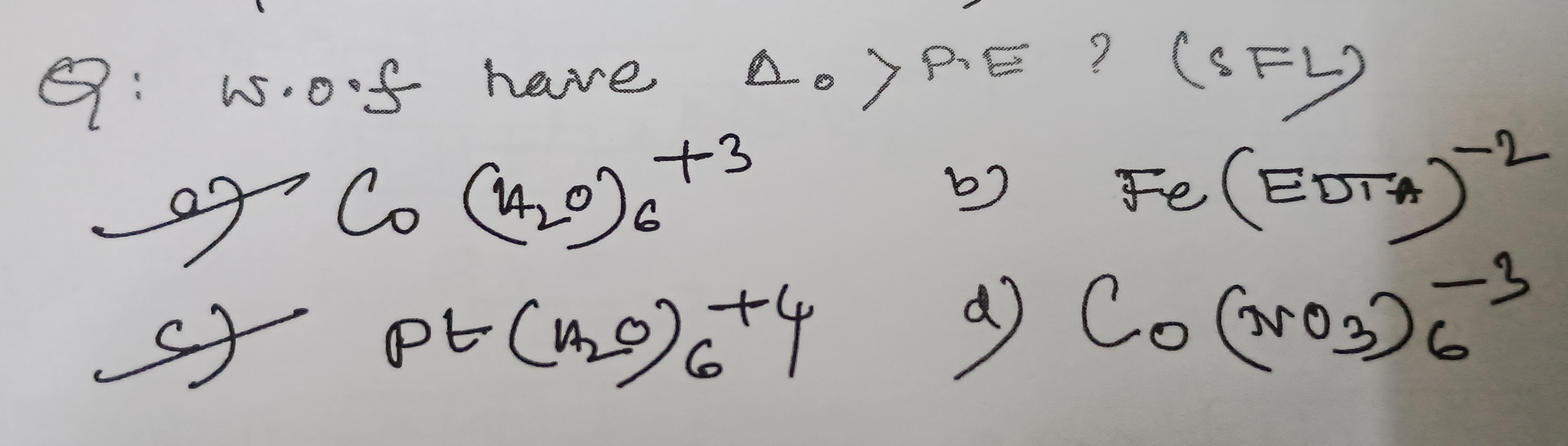
Co(H2O)6+3
Fe(EDTA)−2
Pt(H2O)6+4
Co(NO3)6−3
b) Fe(EDTA)−2 and c) Pt(H2O)6+4
Solution
The condition Δo>P.E signifies a low-spin complex. This occurs when the crystal field splitting energy (Δo) is greater than the electron pairing energy (P.E.). In such complexes, electrons fill the lower energy t2g orbitals before occupying the higher energy eg orbitals, leading to a reduced number of unpaired electrons.
Let's analyze each option:
-
Co(H2O)6+3: Cobalt is in the +3 oxidation state with a 3d6 electronic configuration. Water (H2O) is a weak to moderate field ligand. For Co+3 (3d6), Δo is typically around 18,000 cm−1, while P.E. is approximately 22,000 cm−1. Thus, Δo<P.E, making it a high-spin complex.
-
Fe(EDTA)−2: Iron is in the +2 oxidation state with a 3d6 electronic configuration. EDTA is a strong field ligand. With a strong ligand, Δo is significantly larger than P.E. for Fe+2 (3d6), resulting in a low-spin complex. Therefore, Δo>P.E.
-
Pt(H2O)6+4: Platinum is in the +4 oxidation state with a 5d6 electronic configuration. Although water (H2O) is a weak field ligand, platinum is a 3rd-row transition metal in a high oxidation state (+4). These factors lead to a very large Δo, which is much greater than P.E. This complex is low-spin. Therefore, Δo>P.E.
-
Co(NO3)6−3: Cobalt is in the +3 oxidation state with a 3d6 electronic configuration. Nitrate (NO3−) is a weak field ligand. For Co+3 (3d6) with a weak ligand, Δo is smaller than P.E., resulting in a high-spin complex. Thus, Δo<P.E.
Therefore, the complexes satisfying Δo>P.E are Fe(EDTA)−2 and Pt(H2O)6+4.
