Question
Question: which relation is mcorrect for given rxⁿ?? $PCl_5 (g) \longrightarrow PCl_3 (g) + Cl_2 (g)$...
which relation is mcorrect for given rxⁿ??
PCl5(g)⟶PCl3(g)+Cl2(g)
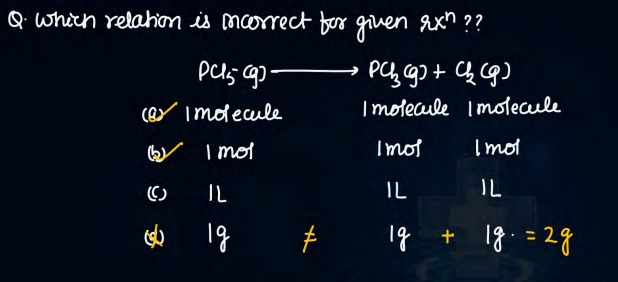
A
1 molecule 1 molecule 1 molecule
B
1 mol 1 mol 1 mol
C
1L 1L 1L
D
1g = 1g + 1g. = 2g
Answer
Options (a) and (b)
Explanation
Solution
For the reaction
PCl5(g)⟶PCl3(g)+Cl2(g)the stoichiometric coefficients are all 1. This means:
-
1 molecule of PCl₅ produces 1 molecule of PCl₃ and 1 molecule of Cl₂
-
In terms of moles, 1 mol of PCl₅ yields 1 mol of PCl₃ and 1 mol of Cl₂
Option (a) expresses the molecular relationship and option (b) expresses the molar relationship.
Option (c) incorrectly equates equal volumes (which is valid only if conditions are identical) without considering the overall reaction yield, and option (d) misrepresents mass relationships.
