Question
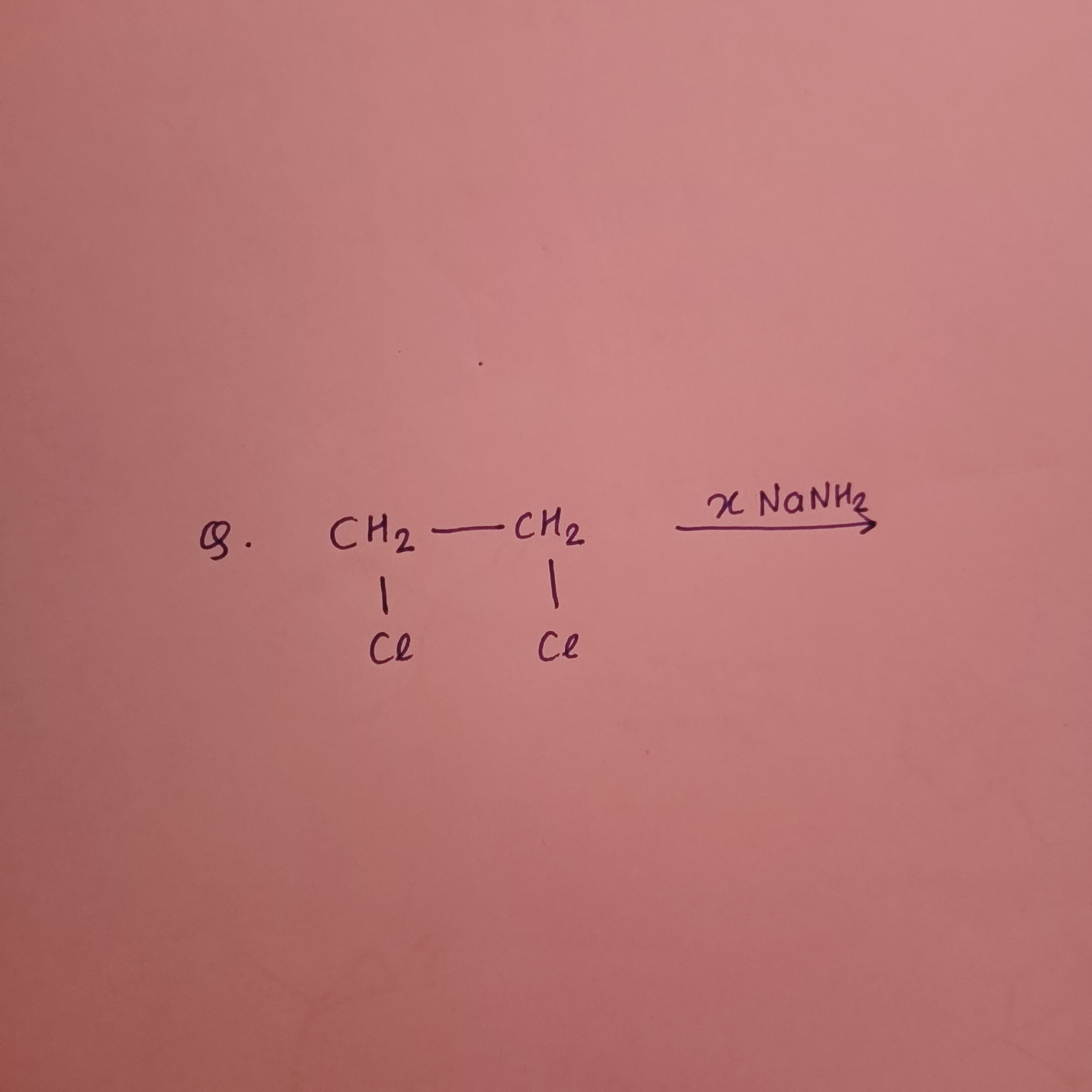
Question: $\qquad CH_2-CH_2 \xrightarrow{xNaNH_2}$ $\qquad | \qquad |$ $\qquad Cl \qquad Cl$ ...
CH2−CH2xNaNH2 ∣∣ ClCl
Answer
Acetylene (Ethyne)
Explanation
Solution
Given the molecule
ClCl∣∣CH2−CH2this is 1,2-dichloroethane. When treated with excess NaNH2, a strong base, two successive E2 eliminations occur (first forming a vinyl chloride intermediate and then an alkyne), ultimately yielding acetylene (ethyne).
Reaction:
ClCH2-CH2ClxNaNH2HC≡CHExcess NaNH2 deprotonates twice, eliminating two HCl molecules from 1,2-dichloroethane to form acetylene.
