Question
Question: For the concn cell $X_{(s)}|X^{+n}_{(aq)}(\frac{M}{20})||X^{+n}_{(aq)}(\frac{M}{2})|X_{(s)}$ $E_{c...
For the concn cell
X(s)∣X(aq)+n(20M)∣∣X(aq)+n(2M)∣X(s)
Ecell=0.029V at 298K then Calculate 'n' ?
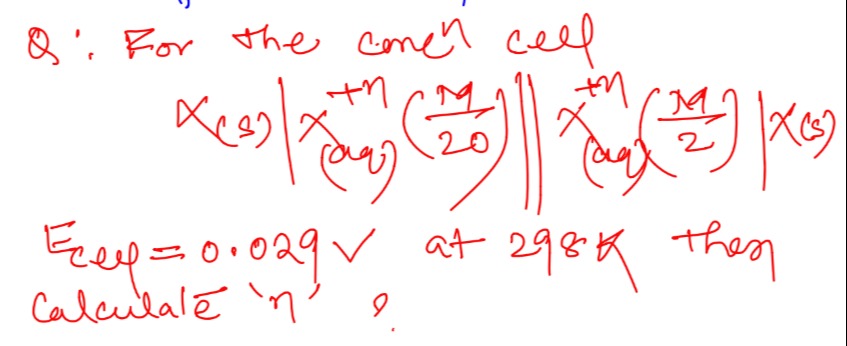
2
Solution
The given cell is a concentration cell:
X(s)∣X(aq)+n(20M)∣∣X(aq)+n(2M)∣X(s)
In a concentration cell, the same electrode material is used, and the electrolyte is the same substance but at different concentrations. The standard cell potential (Ecell∘) for a concentration cell is zero.
The half-reactions are:
Anode (oxidation): X(s)→X(aq)+n(C1)+ne−
Cathode (reduction): X(aq)+n(C2)+ne−→X(s)
Where C1=20M (concentration in the anode compartment) and C2=2M (concentration in the cathode compartment).
For a spontaneous reaction in a concentration cell, the net reaction tends to equalize the concentrations. Thus, the higher concentration will be reduced at the cathode, and the lower concentration will be produced at the anode.
The overall cell reaction is:
X(aq)+n(C2)→X(aq)+n(C1)
The Nernst equation for the cell at 298 K is:
Ecell=Ecell∘−n0.0592logQ
Since Ecell∘=0 for a concentration cell:
Ecell=−n0.0592logQ
The reaction quotient Q for the overall reaction X(aq)+n(C2)→X(aq)+n(C1) is:
Q=[X+n]cathode[X+n]anode=C2C1
Substitute the given concentrations:
C1=20M
C2=2M
Q=M/2M/20=202=101
Now substitute the value of Q into the Nernst equation:
Ecell=−n0.0592log(101)
We know that log(101)=log(10−1)=−1.
So, the equation becomes:
Ecell=−n0.0592(−1)
Ecell=n0.0592
We are given Ecell=0.029 V.
Substitute this value into the equation:
0.029=n0.0592
Now, solve for 'n':
n=0.0290.0592
n≈2.041
Since 'n' represents the number of electrons transferred in the half-reaction (or the charge/valency of the ion), it must be an integer. The calculated value 2.041 is very close to 2. It is common in such problems that the given values or constants are slightly rounded, leading to a result that is approximately an integer.
Therefore, the value of 'n' is 2.
