Question
Question: Calculate the weight of 0.5 mole atoms of sulphur....
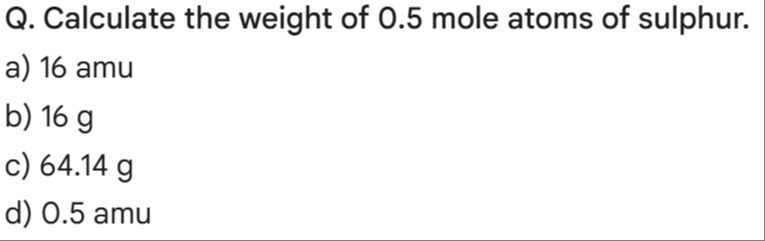
Calculate the weight of 0.5 mole atoms of sulphur.
A
16 amu
B
16 g
C
64.14 g
D
0.5 amu
Answer
16 g
Explanation
Solution
The atomic weight of sulphur is approximately 32 g/mol. Hence, for 0.5 mole atoms:
Weight=0.5×32g/mol=16g