Question
Question: Atomic mass of Ca is not shown by...
Atomic mass of Ca is not shown by
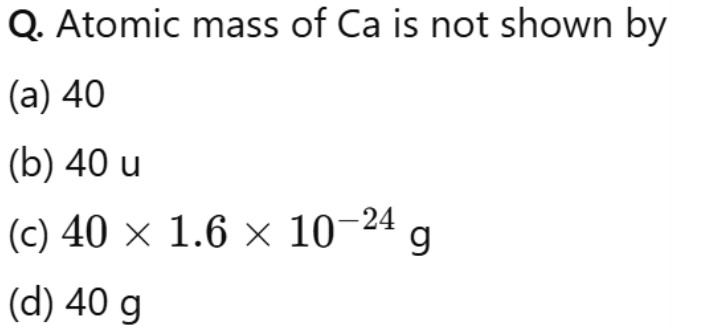
A
40
B
40 u
C
40 × 1.6 × 10^{-24} g
D
40 g
Answer
40 g
Explanation
Solution
Atomic mass of Ca is given as 40 (dimensionless) or 40 u (atomic mass units). Writing it as 40×1.6×10−24g is an approximate conversion of u to grams (though the exact conversion factor is approximately 1.66×10−24g). However, expressing the atomic mass as 40 g is incorrect because 40 g represents the mass of 40 grams, not the mass of a single Ca atom.
