Question
Question: If s-p mixing were not operative, then how many of the following would have different magnetism than...
If s-p mixing were not operative, then how many of the following would have different magnetism than it would with s-p mixing? B2,C2,N2,O2,B22−,B2−,C2−,N2+,O2+
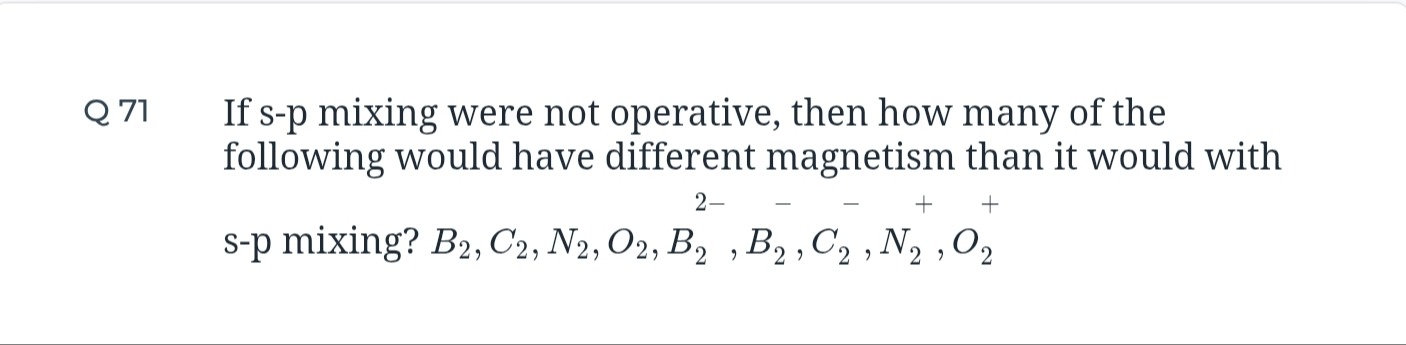
1
Solution
To determine how many of the given species would have different magnetism if s-p mixing were not operative, we need to compare their magnetic properties using two different molecular orbital (MO) energy level orders for diatomic molecules of second-period elements.
1. Understanding s-p mixing: For diatomic molecules of elements from Li2 to N2 (and their ions), the atomic orbitals 2s and 2p mix significantly. This mixing raises the energy of the σ2p orbital above the π2p orbitals.
- MO Order with s-p mixing: σ2s<σ2s∗<π2p<σ2p<π2p∗<σ2p∗
For diatomic molecules of O2, F2, and Ne2 (and their ions), s-p mixing is not significant. The energy order is the standard one where σ2p is lower than π2p.
- MO Order without significant s-p mixing: σ2s<σ2s∗<σ2p<π2p<π2p∗<σ2p∗
The question asks us to compare the magnetism in the scenario with s-p mixing (which is the actual MO diagram for B, C, N) versus the scenario without s-p mixing (which is a hypothetical diagram for B, C, N, but the actual diagram for O, F).
2. Analyzing each species: We will calculate the total number of valence electrons for each species and fill the MOs according to the two orders. A species is paramagnetic if it has unpaired electrons and diamagnetic if all electrons are paired.
The species are: B2,C2,N2,O2,B22−,B2−,C2−,N2+,O2+.
Species with significant s-p mixing (B, C, N and their ions):
- MO Order (with s-p mixing): σ2s<σ2s∗<π2p<σ2p<π2p∗<σ2p∗
- MO Order (without s-p mixing): σ2s<σ2s∗<σ2p<π2p<π2p∗<σ2p∗
Species where s-p mixing is NOT significant (O and its ions):
- The MO order is the same in both scenarios: σ2s<σ2s∗<σ2p<π2p<π2p∗<σ2p∗. Therefore, O2 and O2+ will not show a difference in magnetism.
Let's analyze the remaining species:
-
B2 (6 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^2. Unpaired e⁻: 2 (π2p). Paramagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^2. Unpaired e⁻: 0 (σ2p). Diamagnetic.
- Magnetism difference: YES
-
C2 (8 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^4. Unpaired e⁻: 0. Diamagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^2 \pi_{2p}^2. Unpaired e⁻: 0. Diamagnetic.
- Magnetism difference: NO
-
N2 (10 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^4 \sigma_{2p}^2. Unpaired e⁻: 0. Diamagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^2 \pi_{2p}^4. Unpaired e⁻: 0. Diamagnetic.
- Magnetism difference: NO
-
B22− (6 + 2 = 8 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^4. Unpaired e⁻: 0. Diamagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^2 \pi_{2p}^2. Unpaired e⁻: 0. Diamagnetic.
- Magnetism difference: NO
-
B2− (6 + 1 = 7 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^3. Unpaired e⁻: 1 (π2p). Paramagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^1 \pi_{2p}^2. Unpaired e⁻: 1 (σ2p). Paramagnetic.
- Magnetism difference: NO
-
C2− (8 + 1 = 9 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^4 \sigma_{2p}^1. Unpaired e⁻: 1 (σ2p). Paramagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^2 \pi_{2p}^3. Unpaired e⁻: 1 (π2p). Paramagnetic.
- Magnetism difference: NO
-
N2+ (10 - 1 = 9 valence e⁻)
- With s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \pi_{2p}^4 \sigma_{2p}^1. Unpaired e⁻: 1 (σ2p). Paramagnetic.
- Without s-p mixing: \sigma_{2s}^2 \sigma^*_{2s}^2 \sigma_{2p}^1 \pi_{2p}^4. Unpaired e⁻: 1 (σ2p). Paramagnetic.
- Magnetism difference: NO
Only B2 shows a change in magnetism (from paramagnetic to diamagnetic).
The species that exhibit a change in magnetism are those where the filling of π2p and σ2p orbitals differs in the two scenarios, leading to a different number of unpaired electrons. This occurs for B2.
Summary of Magnetism:
| Species | Valence e⁻ | With s-p mixing | Without s-p mixing | Difference? |
|---|---|---|---|---|
| B2 | 6 | Paramagnetic | Diamagnetic | Yes |
| C2 | 8 | Diamagnetic | Diamagnetic | No |
| N2 | 10 | Diamagnetic | Diamagnetic | No |
| O2 | 12 | Paramagnetic | Paramagnetic | No |
| B22− | 8 | Diamagnetic | Diamagnetic | No |
| B2− | 7 | Paramagnetic | Paramagnetic | No |
| C2− | 9 | Paramagnetic | Paramagnetic | No |
| N2+ | 9 | Paramagnetic | Paramagnetic | No |
| O2+ | 11 | Paramagnetic | Paramagnetic | No |
Only one species, B2, would have a different magnetism.
The final answer is 1.
