Question
Question: Incorrect statement among the following is...
Incorrect statement among the following is
A
All C – C bond lengths in benzene are same
B
All C – C bond lengths in Naphthalene are same
C
Naphthalene has three resonance structures
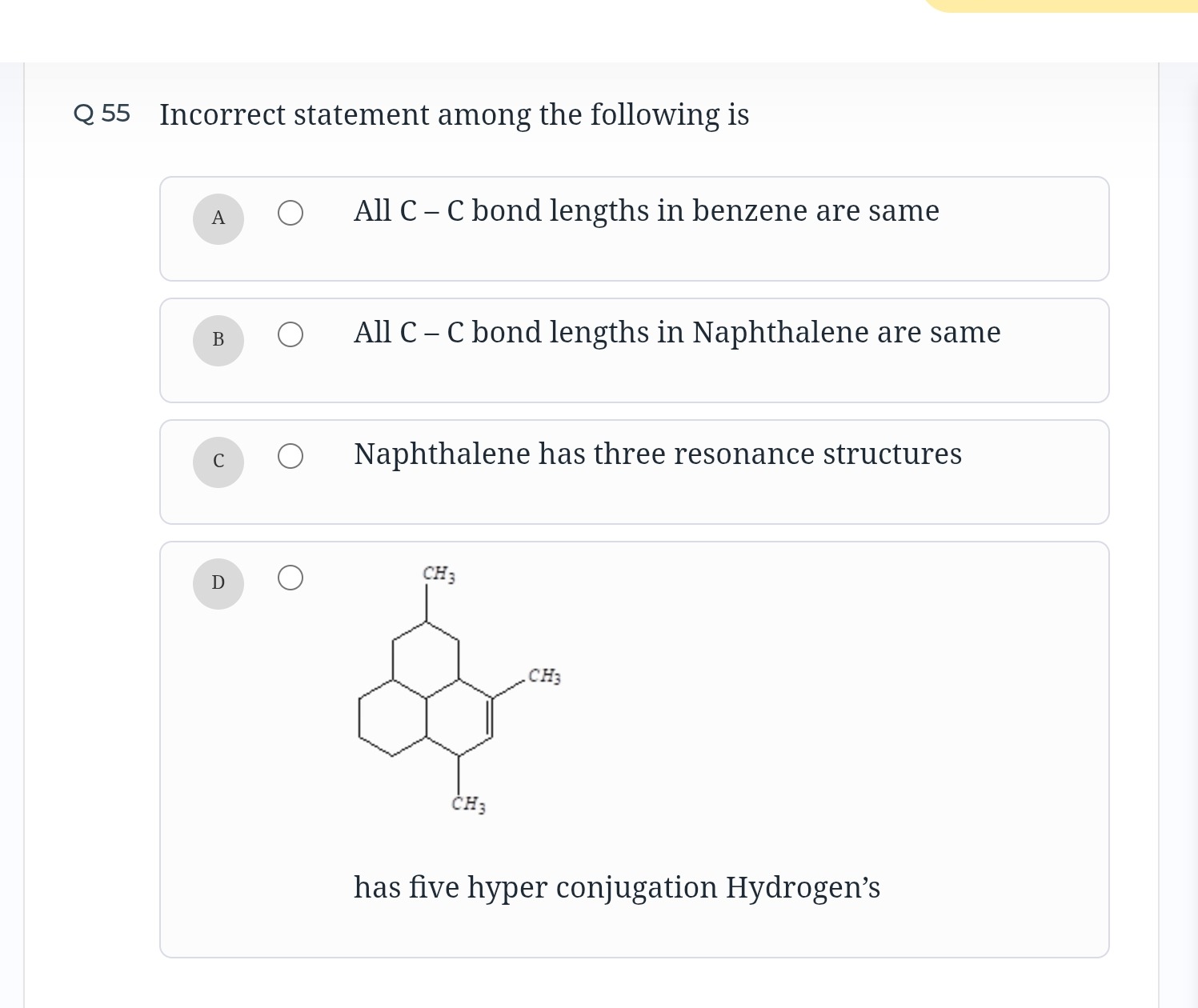
D
has five hyper conjugation Hydrogen's
Answer
All C – C bond lengths in Naphthalene are same
Explanation
Solution
Statement A is correct: Benzene has equivalent C-C bonds due to resonance, all measuring 139 pm.
Statement C is correct: Naphthalene exhibits resonance with three contributing structures.
Statement D is correct: The molecule shown has 5 hyperconjugation hydrogens (3 from the methyl group and 1 each from two adjacent ring carbons).
Statement B is incorrect: Naphthalene has different C-C bond lengths (approx. 136 pm, 139 pm, and 140 pm), not all the same.
