Question
Question: Which carbocation is the most stabilized by resonance....
Which carbocation is the most stabilized by resonance.
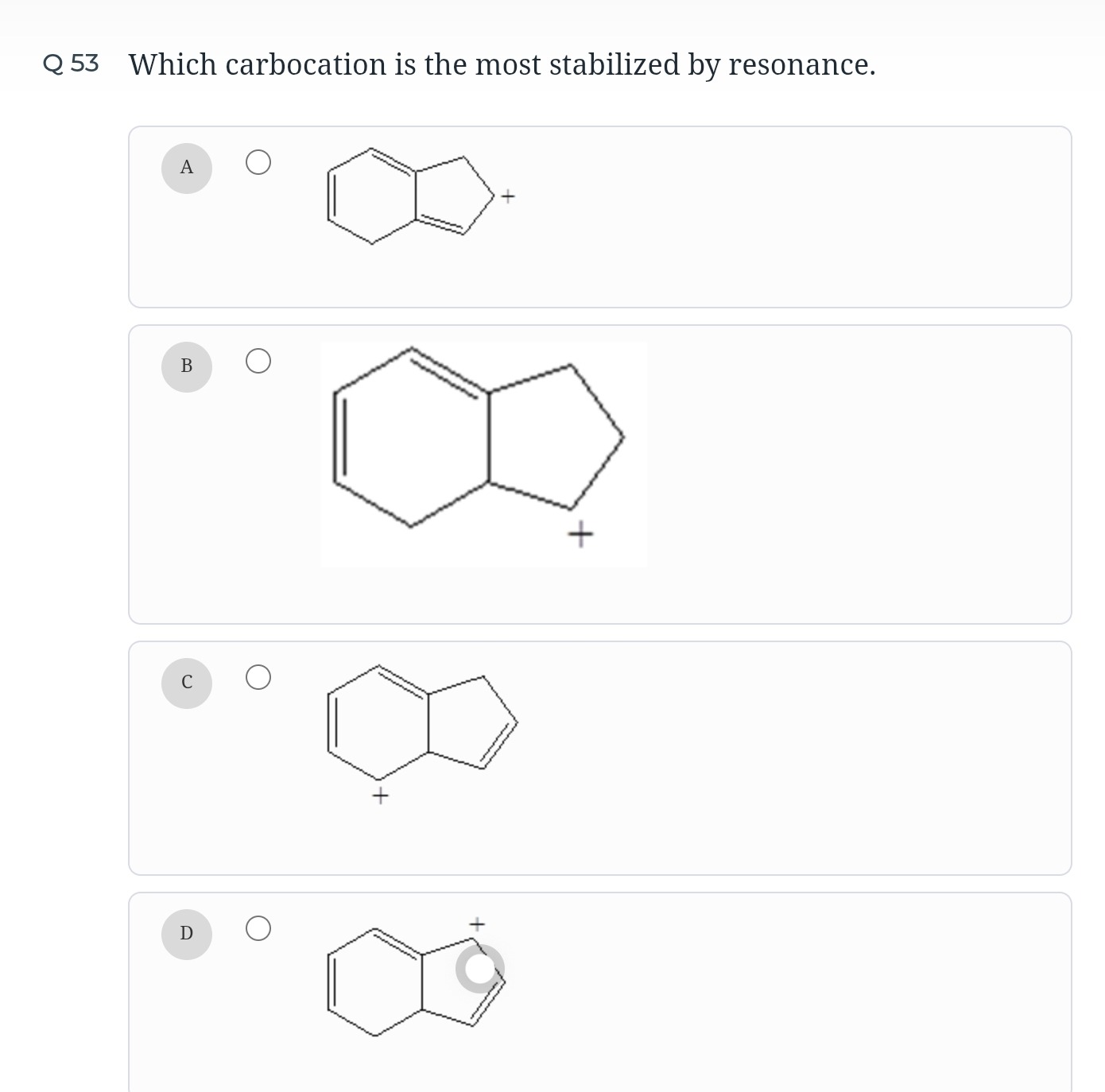
A
A
B
B
C
C
D
D
Answer
D
Explanation
Solution
The stability of carbocations is enhanced by resonance when the positive charge can be delocalized over a conjugated pi system. Options A, C, and D involve allylic carbocations, where the positive charge is adjacent to a double bond, allowing for resonance. Option D is the most stabilized because the positive charge is part of a fused bicyclic system with extended conjugation, leading to a more extensive delocalization of the positive charge over a larger pi system.
