Question
Question: A \xrightarrow{2HCl} . Reactant (A) can be:...
A \xrightarrow{2HCl} . Reactant (A) can be:
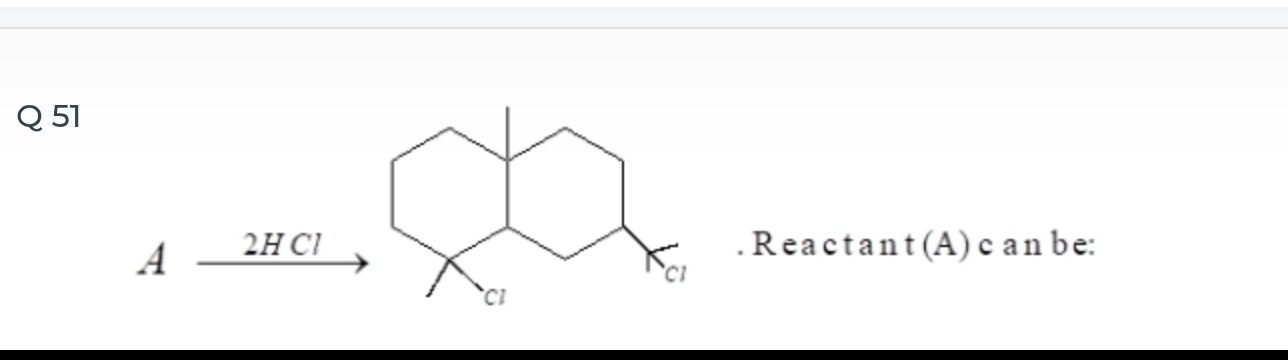
The question is incomplete as the options are not provided. However, based on the reaction shown, the reactant (A) must be a bicyclic diene. A plausible structure for A would be a decalin derivative with two double bonds in one of the rings, leading to the formation of the given dichlorinated product upon addition of 2HCl. For example, if the product is 1-methyl-4,5-dichlorobicyclo[4.4.0]decane, then a possible reactant A could be 1-methylbicyclo[4.4.0]deca-3,5-diene or a related isomer.
Solution
The reaction involves the addition of 2 molecules of HCl to a reactant A, forming a dichlorinated bicyclic compound. This indicates that reactant A is a diene, containing two double bonds. The product shows two chlorine atoms on adjacent carbons in one of the rings. One of these carbons is tertiary, and the other is secondary. By reversing the reaction, we can deduce the positions of the double bonds in A. Removing the two chlorine atoms and adding two double bonds leads to a cyclic diene. Specifically, if we consider the tertiary carbon with a chlorine and the adjacent secondary carbon with a chlorine, and assume they were originally part of a conjugated diene system, then the addition of 2HCl would lead to the observed product. The structure of the reactant A is a bicyclic diene with two double bonds in one of the rings, likely in a conjugated or allylic arrangement, which upon reaction with 2HCl yields the dichlorinated product.
