Question
Question: At the middle of the mercury barometer tube there is a little column of air with the length $l_0$ an...
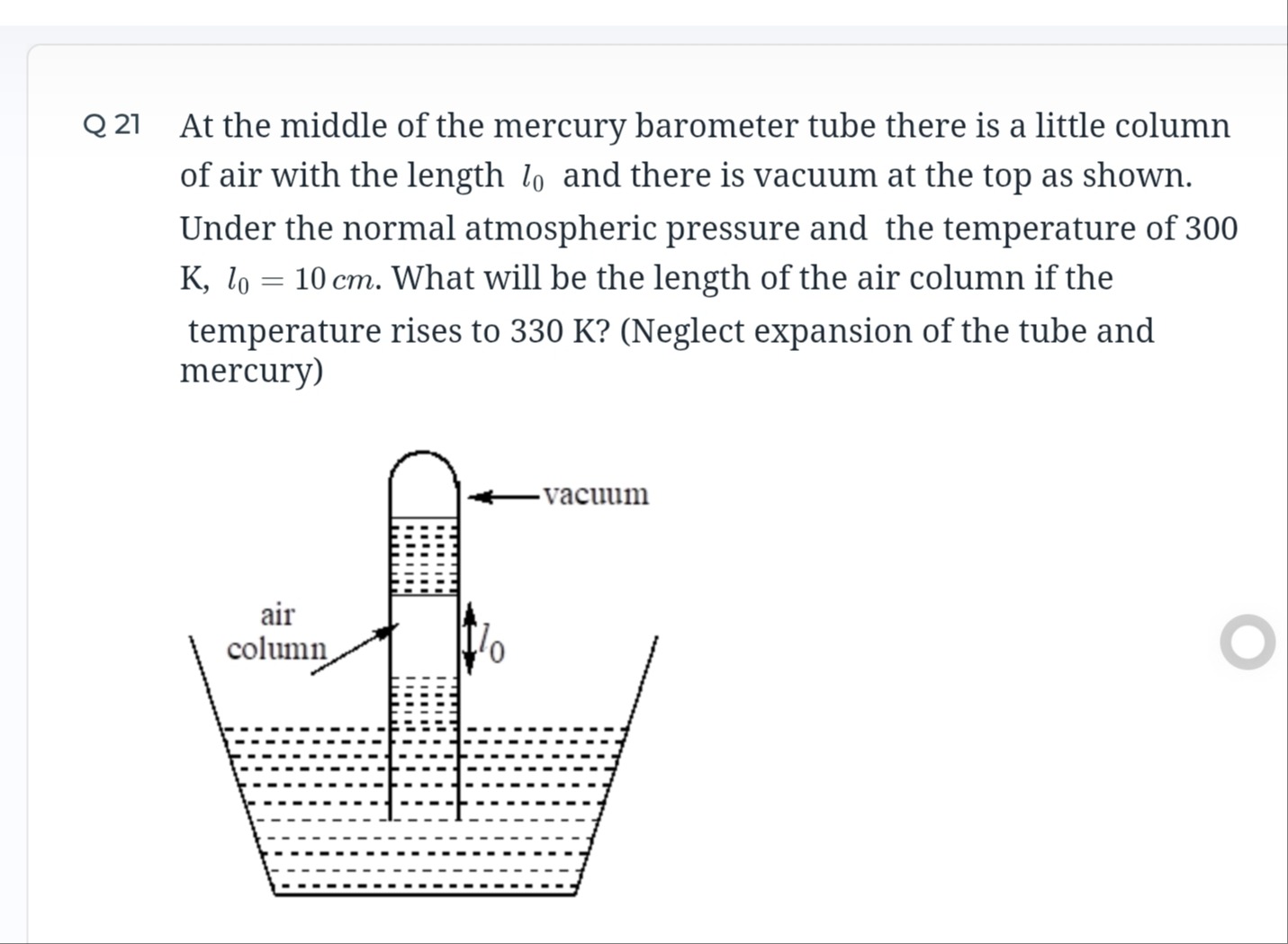
At the middle of the mercury barometer tube there is a little column of air with the length l0 and there is vacuum at the top as shown. Under the normal atmospheric pressure and the temperature of 300 K, l0=10cm. What will be the length of the air column if the temperature rises to 330 K? (Neglect expansion of the tube and mercury)
10 cm
11 cm
12 cm
13 cm
11 cm
Solution
The pressure of the trapped air column is constant because the atmospheric pressure and the height of the mercury column are assumed to be constant. Under constant pressure, the volume of an ideal gas is directly proportional to its absolute temperature. Since the volume of the air column is proportional to its length, the length of the air column is directly proportional to its absolute temperature. Using this relationship, the new length of the air column is calculated: l=l0T0T=10 cm×300 K330 K=11 cm.
